| Citation: | Yong Peng, Yifu Ma, Yaping He, Yuehong Kong, Pengfei Xing, Meiling Xu, Chanchan Shan, Chengliang Zhou, Junjun Zhang, Liyuan Zhang. The Anti-tumor Effect and Immunological Cell Changes of PRaG Therapy in Colon Cancer Peritoneal Metastasis of Mice[J]. Diseases & Research. DOI: 10.54457/DR.202402016 |
To illustrate the anti-tumor effect of PRaG therapy in the peritoneal metastasis of colon cancer in mice and explore the immunological cell changes involved provide reference for PRaG clinical research.
The CT26.WT colon cancer cells were subcutaneously and intraperitoneally inoculated in BALB/c mice, to construct a subcutaneous tumor peritoneal metastasis model. The mice were divided into control group (CON), PD-1 inhibitor group (P), radiotherapy group (Ra), PD-1 inhibitor and radiotherapy group (PRa), PD-1 inhibitor, radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) group (PRaG). Caliper was used to measure the long and short diameter of the subcutaneous tumor to calculate the tumor volume. Ethical death standards were set with tumor volume >1,500 mm3, and the survival period of mice was recorded. Flow cytometry was employed to detect and analyze the differences of T cell percentage and phenotype, and myeloid-derived suppressor cell (MDSCs) subgroups in peripheral blood across different treatment groups.
Compared with CON and P groups, the PRaG group showed significantly reduced subcutaneous tumor volume (P < 0.01). Compared with CON and PRa groups, the PRaG group had more extended survival period (P < 0.05). In peripheral blood, the PRaG group had reduced CD4+ T cell percentage compared with the CON, Ra, and PRa groups (P < 0.05). G-MDSC percentage decreased obviously in PRaG group compared to CON and Ra groups (P < 0.05).
In the subcutaneous tumor peritoneal metastasis model of colorectal cancer, compared with the combined radiotherapy and PD-1 inhibitor, PRaG therapy has more significant anti-tumor effect. The specific cellular mechanisms may be related to the reduction of CD4+ T cells in peripheral blood.
Colorectal cancer ranks third in global cancer incidence and second in mortality[1,2]. Metastasis is the main cause of death in patients with colorectal cancer[3], with the liver, lung, and peritoneum as the most common metastatic sites[4]. Among these, peritoneal metastasis has the worst prognosis, with a median survival period of only 5 to 9 months[5].
The PRaG research team, based on the rules of tumor immune response[6], independently designed a registered clinical trial (registration number: ChiCTR1900026175). It used a triple combination treatment approach involving PD-1 inhibitors, radiotherapy, and granulocyte-macrophage colony-stimulating factor (GM-CSF) to treat advanced solid tumor patients. The acronym from “PD-1 inhibitor, Radiotherapy, and GM-CSF” forms PRaG, hence this combination was named “PRaG Treatment”. The clinical trial included 54 patients with advanced refractory solid tumors, got objective response rate (ORR) of 17% and median progression-free survival (PFS) of 4 months, median overall survival (OS) of 10.5 months[7]. To elucidate the efficacy and cellular mechanisms of PRaG therapy in animal experiments, the study constructed a model of colon cancer subcutaneous tumor peritoneal metastasis. Then the study observed the effect of PRaG therapy on tumor and mice survival and explored related cellular mechanisms.
A total of 112 male BALB/c mice aged 6-8 weeks were purchased from the Experimental Animal Center of Soochow University (Suzhou, China). All experiments were performed in compliance with the rules and regulations for the care and protection of animals as established by the Soochow University Animal Care and Ethics Committee. All animal experiments complied with the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines and were conducted in accordance with the guidelines of the Animal Use and Care of the National Institutes of Health (NIH). CT26.WT colon cancer cell line was purchased from Wuhan Prunus Life Sciences Co., Ltd. Flow cytometry antibodies including CD45-erCP/Cyanine5.5, CD4-FITC, CD8-PE, CD226-APC, CD3-APC-Cy7, Ly-6G-FITC, CD11b-PE, Ly-6C-APC, CD45-APC-Cy7 and TruStain FcX™ PLUS were purchased from Biolegend. Red Blood Cell Lysis Solution (RBC Lysing Buffer) was purchased from BD company. Mice PD-1 monoclonal antibody (clone number: G4C2) was gift from Shanghai Junshi Biosciences Co Ltd. Recombinant mice Granulocyte-Macrophage Colony-Stimulating Factor (rmGM-CSF) was gift from Xiamen Amoytop Biotech Co., Ltd, Xiamen, China.
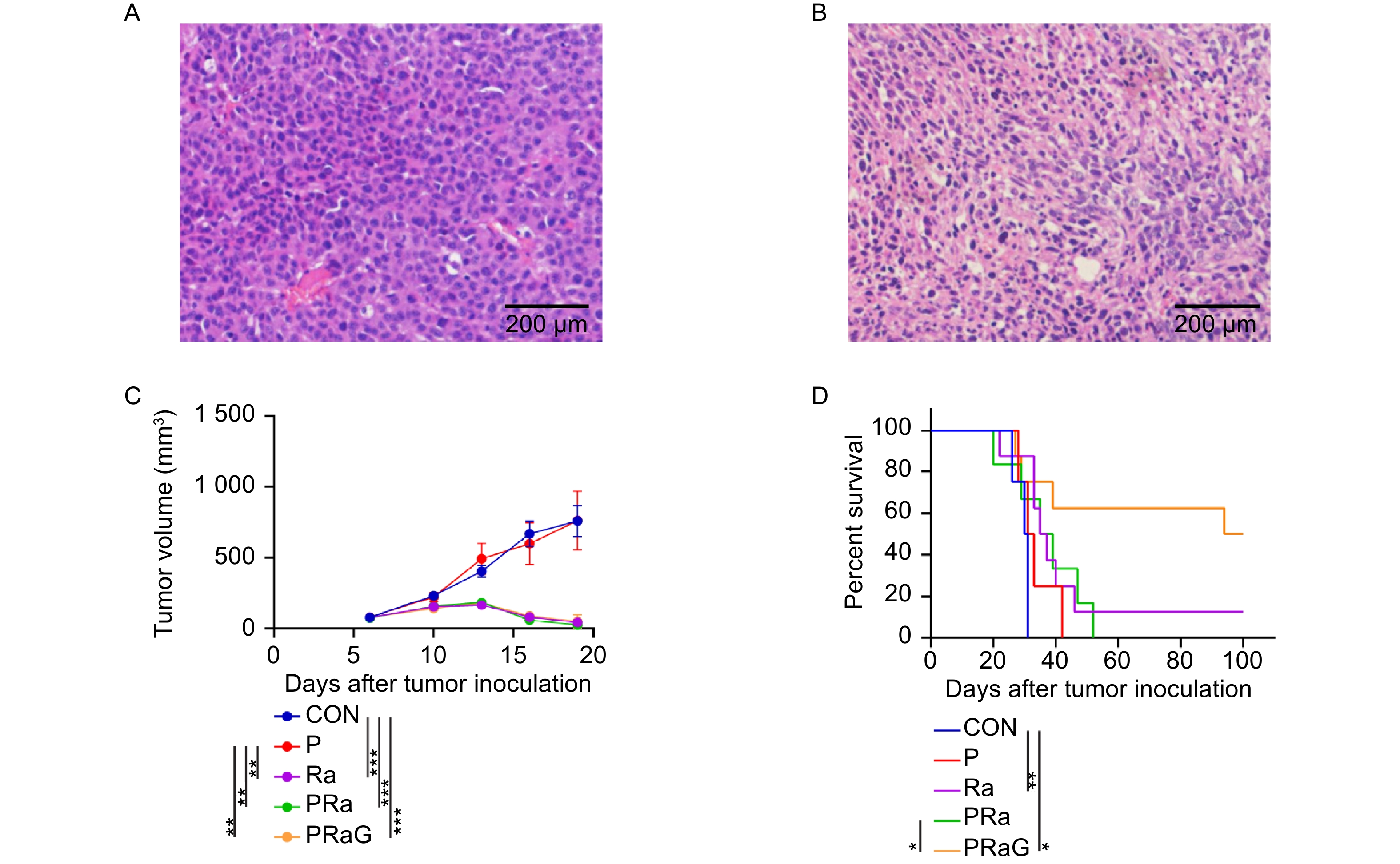
The CT26.WT cells 3 × 105 were inoculated subcutaneously in the right thigh, and 1 × 105 CT26.WT cells were inoculated into the abdominal cavity 5 d later. To further validate the construction of subcutaneous and peritoneal metastasis model of mice, H&E staining was performed on the subcutaneous and peritoneal mass at 12 d after right subcutaneous tumor inoculation (Fig. 1). The staining results showed that the cells represented enlarged nuclei and increased nucle-plasma ratio, obvious nucleolus, and pathological mitotic signs, indicating malignant tumor cells. In summary, the peritoneal metastasis model of subcutaneous tumor of colon cancer was successfully constructed.
At 6 d after the right subcutaneous tumor inoculation, caliper was used to measure the length and width of the subcutaneous tumor and calculate the volume (Volume = 0.5 × length × width2). Groups were evenly distributed based on tumor size. Experiment groups included Control group (CON), PD-1 inhibitor alone group (P), Radiotherapy alone group (Ra), Radiotherapy combined with PD-1 inhibitor group (PRa), and Radiotherapy combined with PD-1 inhibitor and GM-CSF group (PraG). Fifty mice were selected for the examination of tumor volume measurement and divided randomly into five groups including CON, P, Ra, PRa, PRaG (n = 10). Three mice were selected randomly from the above each group for the examination of flow cytometry (n = 3). Then sixty mice were selected for the examination of survival observation and divided randomly into five groups including CON, P, Ra, PRa, PRaG (n = 12). Two mice died from anesthesia.
The subcutaneous tumors were selected for radiotherapy at 7, 8, and 9 d after inoculation. The mice were anesthetized with 1% pentobarbital sodium (7 μL/g) and then placed in prone position. The right subcutaneous tumors were selected as the irradiated area and the non-irradiated area was shielded by lead plate of 1 cm thickness. The low-energy X-ray irradiator (160 kV, 1.1 Gy/min) was employed. Irradiation mode was 8 Gy in 3 fractions. rmGM-CSF was administered at 1 d after radiotherapy, and rmGM-CSF and PD-1 inhibitor on the second day, every 3 d for one cycle, 4 cycles in total. The dosage of rmGM-CSF was 100 ng once per mice, administered via abdominal injection. PD-1 inhibitor dose was 0.25 mg/kg per time, also administered via abdominal injection.
The right subcutaneous tumor was measured at 10, 13, 16, 19 d using caliper for length and width, and the tumor volume was calculated. Ethical death criteria were set at tumor volume > 1,500 mm3, and survival status and time were recorded.
The peripheral blood from the mice was collected at 22 d after right subcutaneous tumor inoculation and was add into red blood cell lysis solution. Then the solution was incubated for 10 min and centrifuged at 1,800 r/min for 5 min. Then the supernatant was discarded and the precipitate was resuspended in 2 mL complete culture medium, and 120 μL cell suspension was taken for flow cytometry antibody staining. The blocking antibody was incubated (1:100) at 4 ℃ for 15 min, membrane antibody (1:1,000) at 4 ℃ for 25 min.
SPSS 16.0 and GraphPad Prism 8.3 were utilized for statistical analysis. Data are presented as mean ± standard error of the mean (mean ± SEM). One-way ANOVA was used for two or more samples, and survival period is analyzed using log-rank test. P < 0.05 was considered statistically significant.
To verify the construction of subcutaneous and peritoneal metastasis model of mice, the histopathological images of the subcutaneous and peritoneal mass were respectively shown as follows (Fig. 1A, B). The tumor volume of the P group was similar to that of the CON group (Fig. 1C, P > 0.05). Compared to the CON group, the tumor volume in the Ra, PRa, PRaG groups reduced (Fig. 1C, P < 0.001). Compared to the P group, the tumor volume in the Ra, PRa, PRaG groups obviously reduced (Fig. 1C, P < 0.01). But there was no statistical difference in tumor volume among Ra, PRa, PRaG groups (Fig. 1C, P > 0.05). Compared to the CON group, survival time was longer in Ra and PRaG groups (Fig. 1D, Ra: P < 0.01, PRaG: P < 0.05). There was no statistical difference in survival time between the CON and PRa group (Fig. 1D, P > 0.05). Compared to the PRa group, survival time increased in PRaG group (Fig. 1D, P < 0.05).
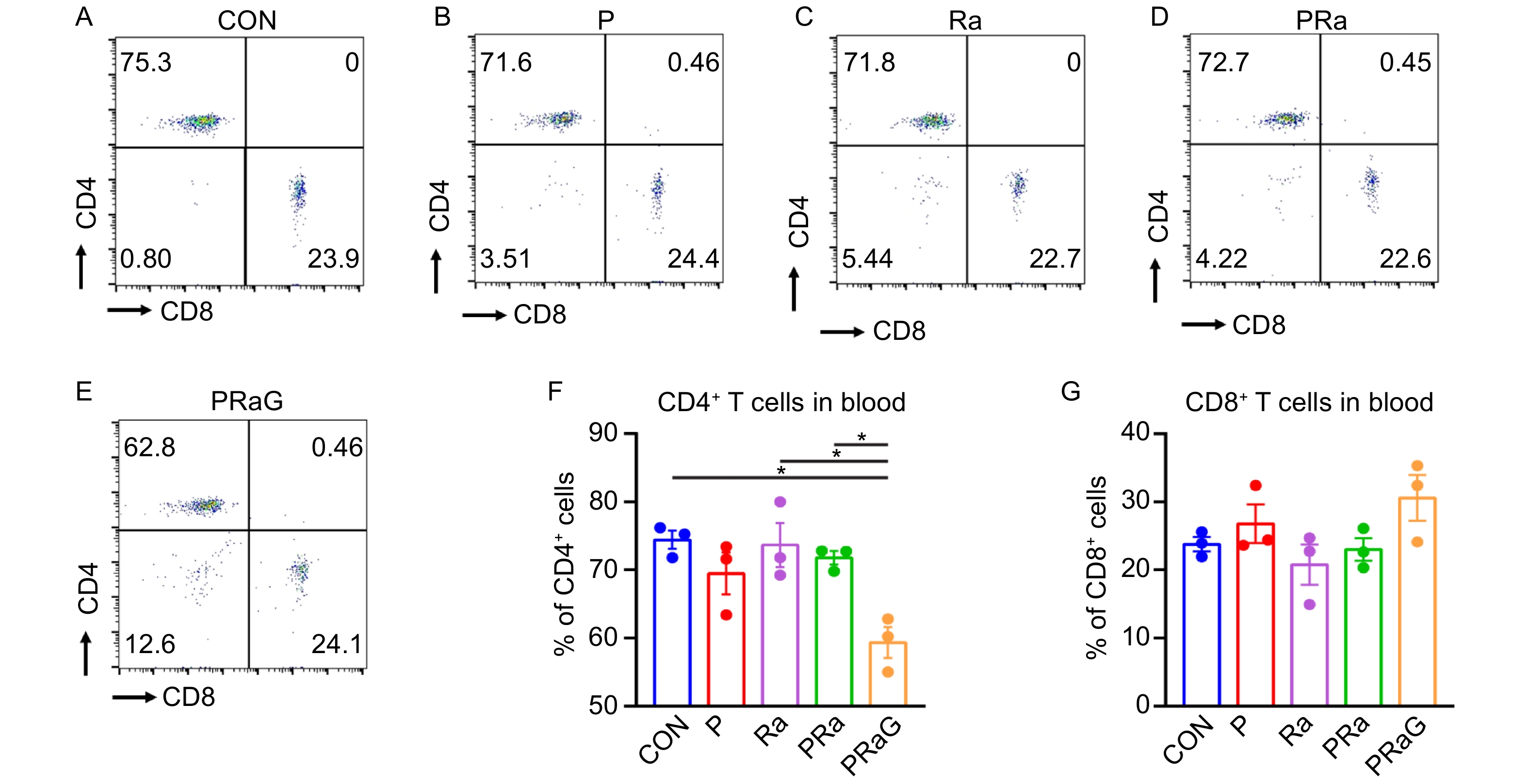
The changes of peripheral blood CD4+ T cell and CD8+ T cell percentages with flow cytometry antibody staining were shown in Fig. 2A-E. Compared to the CON, Ra, PRa group, the CD4+ T cell percentage decreased (Fig. 2F, P < 0.05) in the PRaG group. CD8+ T cell percentage slightly increased compared with other groups, but it had no statistical significance (Fig. 2G, P > 0.05).
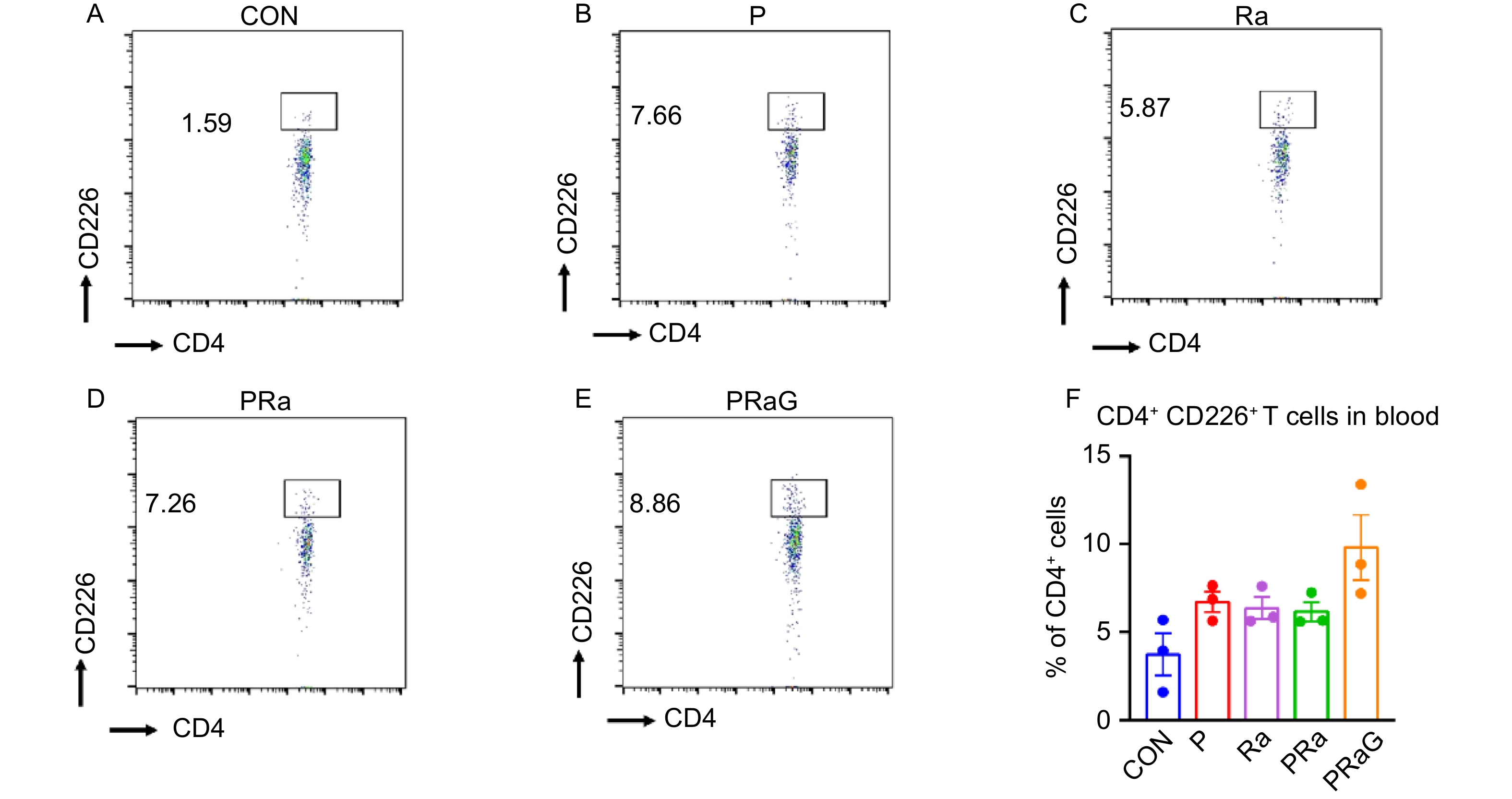
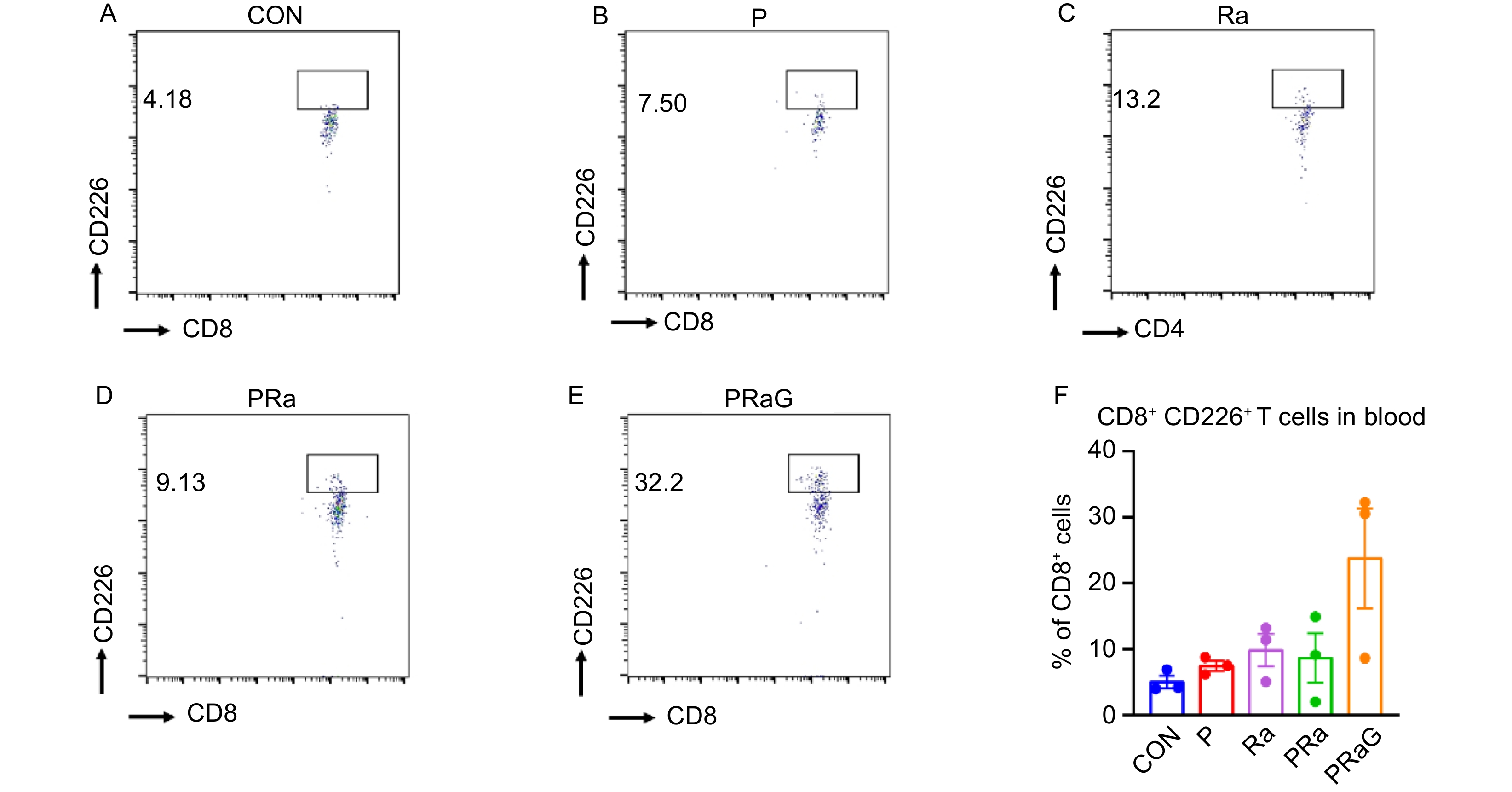
The changes of peripheral blood functional T cell percentages with flow cytometry antibody staining were shown in Fig. 3A-F and Fig. 4A-F. Compared to other groups, both the CD4+ CD226+ T cells and CD8+ CD226+ T cell increased in PRaG group, but without statistical significance (Fig. 3F, Fig. 4F, P > 0.05).
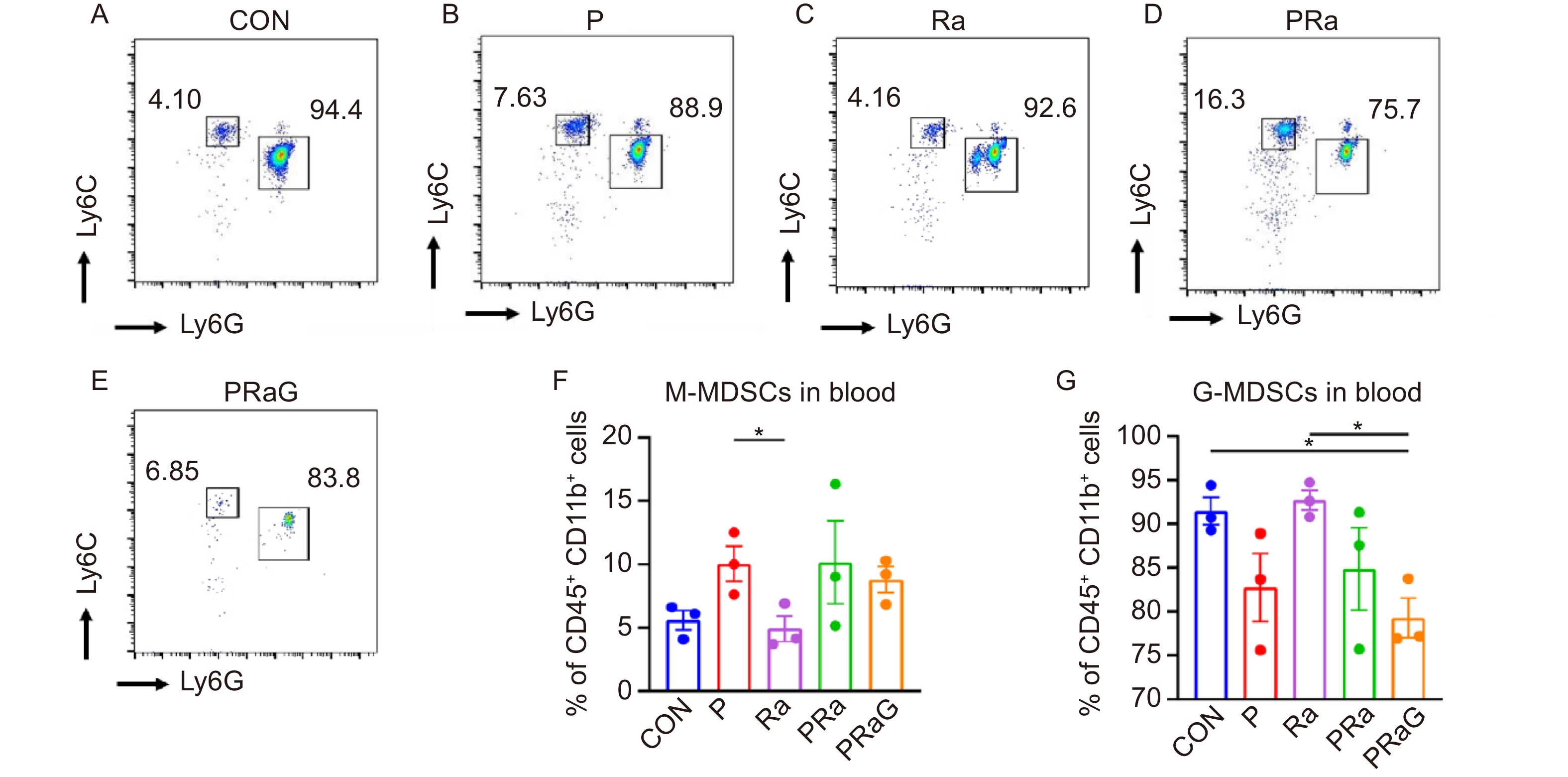
The changes of peripheral blood M-MDSCs and G-MDSCs percentages with flow cytometry antibody staining were shown in Fig. 5A-E. Compared to the P group, the Ra group has a decreased percentage of M-MDSCs (Fig. 5F, P < 0.05). Compared to the CON and Ra groups, the PRaG group has a decreased percentage of G-MDSCs (Fig. 5G, P < 0.05).
Immune checkpoint inhibitor has changed the traditional landscape of colon cancer treatment. Among them, PD-1 inhibitor has become a common drug for colon cancer immunotherapy. However, PD-1 inhibitor is effective only in MSI-H type colon cancer patients, who account for 4-5% of advanced colon cancer patients[8,9]. Granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes the differentiation, maturation, and expansion of dendritic cells (DCs) and triggers DCs to recognize process, and present tumor antigens to T cells, which further enhances the anti-tumor immunity triggered by radiotherapy. In a proof-of-principle trial, a combination of GM-CSF and radiotherapy resulted in abscopal response in 4 of the 11 NSCLC patients, including 2 complete abscopal responses. Additionally, preclinical and clinical studies showed enhanced anti-tumor response by the treatment of PD-1 inhibitors and GM-CSF via promoting T cell infiltration. Therefore, our research employed a combination of PD-1 inhibitor, radiotherapy, and GM-CSF, named PRaG therapy. The clinical trial included 54 patients with advanced refractory solid tumors showed the median PFS of 4 months and the median OS of 10.5 months. In contrast, a study of PD-1 inhibitor combined with radiotherapy conducted by the Chicago University had median PFS and OS of only 3.1 months and 9.6 months respectively[10]. Compared with the above study, PRaG therapy achieved better clinical benefits perhaps related to GM-CSF, the mechanisms of which deserved further exploration.
Subsequently, our study established a subcutaneous tumor peritoneal metastasis model of CT26 cell line representing MSS-type colon cancer. Genetic depletion of Ythdf1 augmented antitumor immunity in CT26 (MSS-CRC)[11]. Combined IL-17A and PD-1 blockade had efficacy in CT26 tumor (MSS-CRC)[12]. WT colon cancer to further validate the efficacy of PRaG therapy and initially explore its cellular mechanisms. The results showed that, compared to radiotherapy combined with PD-1 inhibitor, PRaG therapy significantly extended mice survival time. PRaG therapy is a kind of immunotherapy based on PD-1 inhibitor, and its efficacy is closely related to immune cells. Moreover, increasing evidence suggests that circulating immune cells have a significant impact on tumor prognosis[13]. The phenotype of circulating immune cell subgroups may reflect local immune responses in the tumor microenvironment, providing potentially important information about colon cancer disease progression[14]. Therefore, our study examined changes of the relevant immune cells in mice peripheral blood.
After PRaG therapy, the percentage of CD4+ T cells in mice peripheral blood significantly reduced. CD4+ T cells play an important role in maintaining CD8+ T cell immune responses[15]. However, CD4+ T cells also include Treg, a type of cell with immune-suppressive function. It is unclear which specific CD4+ T cell subgroup is affected by PRaG therapy, which deserves further exploration.
CD226 is an adhesion molecule that can enhance NK cell and CD8+ T cell function. Melanoma patients with a higher proportion of CD226+ T cell subgroups in tumor tissue have correspondingly prolonged PFS[16]. Some research has found that the concentration of soluble CD226 in tumor patient serum was higher than healthy people, while the CD226 expression in PBMC surface is lower than healthy people. The soluble CD226 in peripheral blood may originate from PBMC. Therefore, serum soluble CD226 may be a potential biomarker for predicting the efficacy of tumor immunotherapy[17]. Researchers further found that the soluble CD226 level in peripheral blood increased in skin T cell lymphoma patients, originating from the surface of NK cells and CD8+ T cell[18]. In our study, we have not yet detected the significant changes of soluble CD226 content in mice peripheral blood. Both of CD4+ and CD8+ T cell contained multiple subtypes. After PRaG therapy, the CD226 expression in CD4+ and CD8+ T cell surface was upregulated and possibly derived from peripheral blood soluble CD226, which may explain the better anti-tumor effect of PRaG therapy than other groups. In peripheral blood, the ratio of soluble CD226 accounts for CD226 molecules in cell surface may become a marker for predicting the efficacy of PRaG therapy, which is worth further exploration.
Previous research has discovered that melanoma patients have significantly higher M-MDSCs counts in peripheral blood than normal individuals. Patients with fewer peripheral blood M-MDSCs experienced prolonged survival after anti-CTLA-4 treatment[19]. NSCLC patients experienced a decreased G-MDSCs in peripheral blood after receiving nivolumab treatment. Our study found that after PRaG therapy, the proportion of M-MDSCs in mice peripheral blood increased while G-MDSCs decreased. The correlation between MDSC subgroups and PRaG therapy efficacy warrants further investigation. The changes in MDSC subgroups of mice peripheral blood may also be related to GM-CSF. GM-CSF can promote hematopoietic stem cells to differentiate into multinucleated neutrophils, monocytes, macrophages, and dendritic cells[20−24]. Numerous studies found that GM-CSF has both anti-tumor and pro-tumor effects. In patients with colorectal cancer, tumor cell-derived GM-CSF is positively correlated with prognosis[25]. However, in other types of cancer, such as bladder cancer, malignant glioma, and neck malignancy, GM-CSF expression correlates negatively with prognosis[26−29]. The specific role of GM-CSF in PRaG therapy still requires further study.
In summary, in the subcutaneous tumor peritoneal metastasis model of mice colon cancer, PRaG therapy exhibited more apparent anti-tumor effects compared to radiotherapy combined with PD-1 inhibitor. Its cellular mechanisms may be related to changes of CD4+ T cells and CD226+ CD8+ T cells, which will provide reference for PRaG clinical research. In peripheral blood, the CD226 molecule in cell surface may become a marker for predicting the efficacy of PRaG therapy. Meantime, the specific role of GM-CSF in PRaG therapy still requires further study. The efficacy of PRaG therapy needs increased sample size and more kinds of tumor models in future study.
CTLA-4, Cytotoxic T Lymphocyte-Associated Antigen-4; GM-CSF, Granulocyte-Macrophage Colony Stimulating Factor; G-MDSCs, Granulocytic Myeloid-Derived Suppressor Cells; MDSCs, Myeloid-Derived Suppressor Cells; M-MDSCs, Monocytic Myeloid-Derived Suppressor Cells; MSI-H, Microsatellite instability-High; NSCLC, Non-small cell lung cancer; ORR, Objective Response Rate; OS, overall survival; PD-1, programmed cell death protein 1; PFS, progression free survival.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
YP and YFM, YPH performed the experiments and analyzed the data; YHK and PFX performed imaging analysis; MLX and CCS, CLZ helped flow cytometry data analysis; JJZ and LYZ contributed to study conception and design, project administration, funding acquisition, revised the manuscript and figures; All authors reviewed and approved the final version of the manuscript.
| [1] |
Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin, 2022, 72(1): 7-33. DOI: 10.3322/caac.21708
|
| [2] |
Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin, 2022, 72(5): 409-436. DOI: 10.3322/caac.21731
|
| [3] |
Pretzsch E, Bösch F, Neumann J, et al. Mechanisms of metastasis in colorectal cancer and metastatic organotropism: hematogenous versus peritoneal spread. J Oncol, 2019, 2019: 7407190. DOI: 10.1155/2019/7407190
|
| [4] |
Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep, 2016, 6: 29765. DOI: 10.1038/srep29765
|
| [5] |
Gómez-portilla A, Cendoya I, López TI, et al. Principles of the treatment of peritoneal carcinomatosis due to colorectal cancer Current review and update. Cir Espan, 2005, 77(1): 6-17. DOI: 10.1016/s0009-739x(05)70796-x
|
| [6] |
Kong Y, Ma Y, Zhao X, et al. Optimizing the treatment schedule of radiotherapy combined with anti-PD-1/PD-L1 immunotherapy in metastatic cancers. Front Oncol, 2021, 11: 638873. DOI: 10.3389/fonc.2021.638873
|
| [7] |
Kong Y, Zhao X, Xu M, et al. PD-1 inhibitor combined with radiotherapy and gm-csf (prag) in patients with metastatic solid tumors: an open-label phase ii study. Front Immunol, 2022, 13: 952066. DOI: 10.3389/fimmu.2022.952066
|
| [8] |
Overman MJ, Mcdermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (Check-Mate 142): an open-label, multicentre, phase 2 study. Lancet Oncol, 2017, 18(9): 1182-1191. DOI: 10.1016/S1470-2045(17)30422-9
|
| [9] |
Le DT, Uram JN, Wang H, et al. Blockade in tumors with mismatch-repair deficiency. N Engl J Med, 2015, 372(26): 2509-2520. DOI: 10.1056/NEJMoa1500596
|
| [10] |
Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol, 2018, 36(16): 1611-1618. DOI: 10.1200/JCO.2017.76.2229
|
| [11] |
Bao Y, Zhai J, Chen H, et al. Targeting m6A reader YTHDF1 augments anti-tumour immunity and boosts anti-PD-1 efficacy in colorectal cancer. Gut, 2023, 72(8): 1497-1509. DOI: 10.1136/gutjnl-2022-328845
|
| [12] |
Liu C, Liu R, Wang B, et al. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer, 2021, 9(1): e001895. DOI: 10.1136/jitc-2020-001895
|
| [13] |
Rana AP, Kaur M, Zonunsanga B, et al. Preoperative peripheral blood count in breast carcinoma: predictor of prognosis or a routine test. Int J Breast Cancer, 2015, 2015: 964392. DOI: 10.1155/2015/964392
|
| [14] |
Rocca YS, Roberti MP, Arriaga JM, et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun, 2013, 19(1): 76-85. DOI: 10.1177/1753425912453187
|
| [15] |
Janssen EM, Lemmens EE, Wolfe T, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature, 2003, 421(6925): 852-856. DOI: 10.1038/nature01441
|
| [16] |
Braun M, Aguilera AR, Sundarrajan A, et al. CD155 on tumor cells drives resistance to immunotherapy by inducing the degradation of the activating receptor CD226 in CD8+ T cells. Immunity, 2020, 53(4): 805-823. DOI: 10.1016/j.immuni.2020.09.010
|
| [17] |
Xu Z, Zhang T, Zhuang R, et al. Increased levels of soluble CD226 in sera accompanied by decreased membrane CD226 expression on peripheral blood mononuclear cells from cancer patients. BMC Immunol, 2009, 10: 34. DOI: 10.1186/1471-2172-10-34
|
| [18] |
Takahashi N, Sugaya M, Suga H, et al. Increased soluble CD226 in sera of patients with cutaneous T-cell lymphoma mediates cytotoxic activity against tumor cells via CD155. J Invest Dermatol, 2017, 137(8): 1766-1773. DOI: 10.1016/j.jid.2017.03.025
|
| [19] |
Kitano S, Postow MA, Ziegler CG, et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res, 2014, 2(8): 812-821. DOI: 10.1158/2326-6066.CIR-14-0013
|
| [20] |
Zhan Y, Lew AM, Chopin M. The pleiotropic effects of the gm-csf rheostat on myeloid cell differentiation and function: more than a numbers game. Front Immunol, 2019, 10: 2679. DOI: 10.3389/fimmu.2019.02679
|
| [21] |
Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem, 1977, 252(6): 1998-2003. DOI: 10.1016/S0021-9258(18)71855-3
|
| [22] |
Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood, 1986, 67(2): 257-267. DOI: 10.1182/blood.V67.2.257.257
|
| [23] |
Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med, 1992, 176(6): 1693-1702. DOI: 10.1084/jem.176.6.1693
|
| [24] |
Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods, 1999, 223(1): 77-92. DOI: 10.1016/S0022-1759(98)00204-X
|
| [25] |
Nebiker CA, Han J, Eppenberger-castori S, et al. GM-CSF production by tumor cells is associated with improved survival in colorectal cancer. Clin Cancer Res, 2014, 20(12): 3094-3106. DOI: 10.1158/1078-0432.CCR-13-2774
|
| [26] |
Perez FA, Fligner CL, Yu EY. Rapid clinical deterioration and leukemoid reaction after treatment of urothelial carcinoma of the bladder: possible effect of granulocyte colony-stimulating factor. J Clin Oncol, 2009, 27(34): e215-e217. DOI: 10.1200/JCO.2009.22.4931
|
| [27] |
Wetzler M, Estrov Z, Talpaz M, et al. Granulocyte-macrophage colony-stimulating factor as a cause of paraneoplastic leukaemoid reaction in advanced transitional cell carcinoma. J Intern Med, 1993, 234(4): 417-420. DOI: 10.1111/j.1365-2796.1993.tb00765.x
|
| [28] |
Albulescu R, Codrici E, Popescu ID, et al. Cytokine patterns in brain tumour progression. Mediat Inflamm, 2013, 2013: 979748. DOI: 10.1155/2013/979748
|
| [29] |
Ninck S, Reisser C, Dyckhoff G, et al. Expression profiles of angiogenic growth factors in squamous cell carcinomas of the head and neck. Int J Cancer, 2003, 106(1): 34-44. DOI: 10.1002/ijc.11188
|