| Citation: | Omkar Khade, Sagar Nagrekar, Vivek Parab, Asmita Choubey. Liquid Biopsies: As an Emerging Tool in Cancer Diagnosis and Monitoring Therapies in Metastasis[J]. Diseases & Research, 2023, 3(1): 41-54. DOI: 10.54457/DR.202301007 |
Despite the availability of advanced diagnosis and treatment methods, cancer is still a leading cause of morbidity. The most readily and routinely available method for cancer diagnosis is tissue biopsy. Tissue biopsies are generally based on molecular profiling of primary tumors, which can change during the metastasis stage. Therefore, it’s unable to give sufficient information about the tumor. These problems can be overcome by liquid biopsies which is considered as fast, non-invasive, and more sensitive method for cancer diagnosis. It also has applications in early diagnosis and real-time monitoring of tumor prognosis and development. This can be achieved by molecular profiling of circulating tumor cells (CTCs), cell-free DNA (cfDNA), exosomes, microRNA (miRNA), and tumor-educated platelets (TEPs) from biological fluids which are considered as biomarkers. The expression of these biomarkers gives an entire snapshot of both the primary and metastasis stages of cancer. It is very useful to determine different stages of cancer and to modify treatment strategies based on treatment response. Therefore, it opens up an opportunity to use liquid biopsies in the area of precision medicine. This review summarizes in-depth information on liquid biopsies, cancer metastasis, various methods in liquid biopsies, biomarkers such as CTCs, cfDNA, miRNA, exosomes, TEPs, and the role of these biomarkers in monitoring therapy.
A standard procedure to obtain a resected tumor for profiling involves tissue biopsy or percutaneous biopsy, an invasive surgery that makes acquiring a quality tumor sample cumbersome. Invasive procedures pose a challenge during cancer treatment in the case of metastasis. This is because real-time monitoring is difficult in such procedures, while tumor cells spread to distant organs and might evolve and acquire resistance to therapy in response to the treatment. An invasive tissue biopsy can require a longer time and high cost for sample isolations since tissue biopsy can involve organ penetration, and repeated surgeries are not feasible[1,2]. It can be minimized by the analysis of biological fluids for the profiling of cancer. This non-invasive technique is known as liquid biopsy (LB) it is a repeatable, sensitive, and convenient diagnostic method that can be used. The samples analyzed during the diagnosis mainly include blood samples obtained using Phlebotomy. Other body fluids sampled include cerebrospinal fluid (CSF), urine samples, and pleural effusions[3]. A liquid biopsy involves obtaining peripheral venous blood from the median cubital vein (Vena media cubiti) and isolating circulating tumor markers such as cell-free nucleic acids and circulating tumor cells (CTCs). Apoptotic tumor cells release biomarkers into circulation, including circulating tumor DNA (ctDNA), cell-free RNA (cfRNA), and exosomes. This ctDNA, ctRNA, and exosomes along with CTCs can be detected in the venous blood sample and analyzed for screening and diagnosis of different cancers[4]. The liquid biopsy technology improves the detection of recurring cancer for better monitoring and efficient cancer therapy. Liquid biopsy can profoundly impact the diagnosis, screening, and management of cancer while potentially reducing the risk of other complications, such as hemorrhage or puncture wounds which could lead to infection[5].
Most solid tumors are either curable or at least manageable if they are diagnosed and treated before metastasis occurs. This is possible because of recent advances in the early detection and treatment of cancers. However, cancer that detaches from the primary tumor site and spreads is likely to be fatal[6].
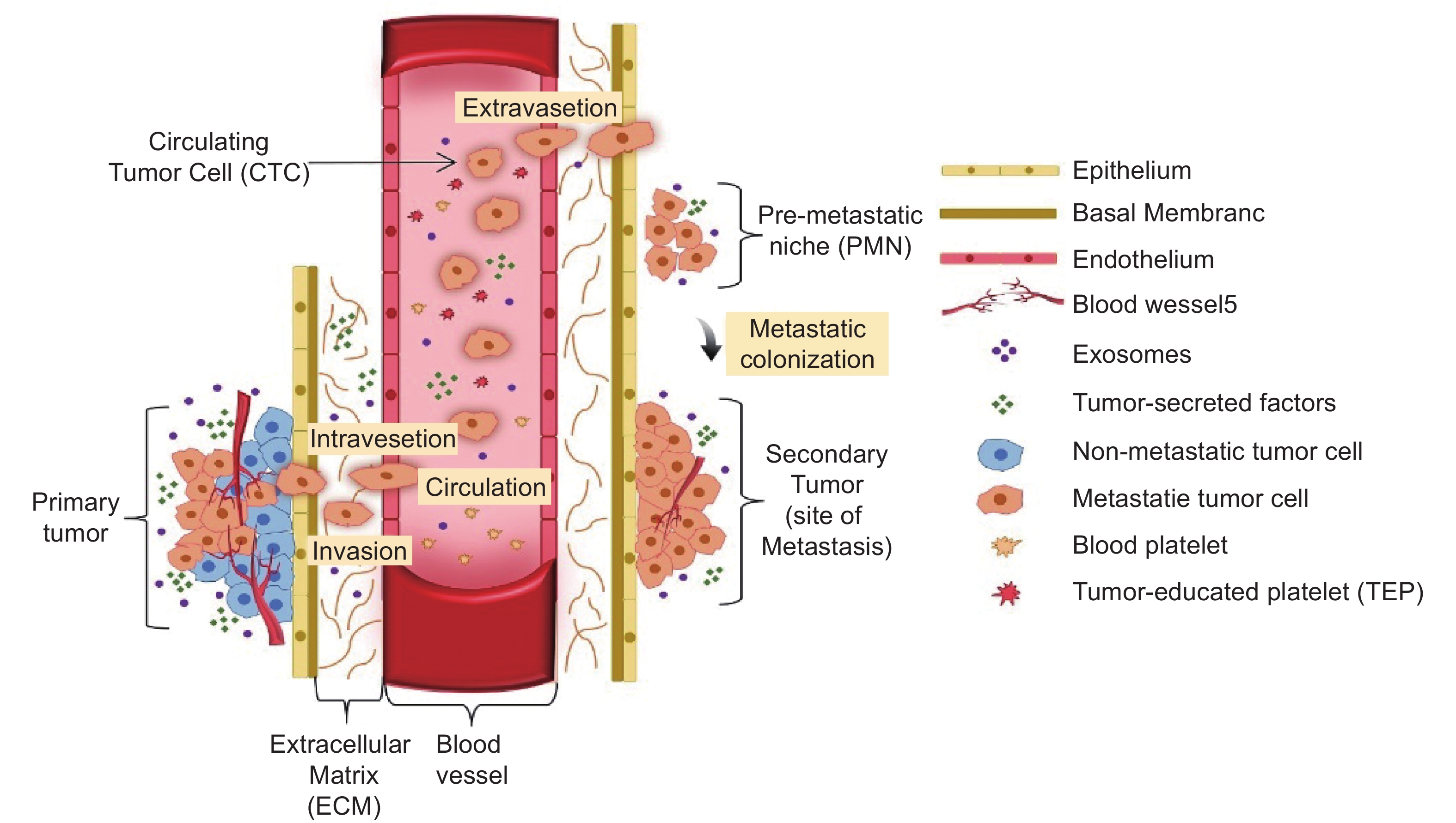
Cancer metastasis is a process in which cancer cells disseminate and migrate from the primary tumor to other sites in the body and grow (Fig. 1). This dissemination occurs through the circulatory and lymphatic systems. About 90% of cancer deaths are primarily caused by metastasis[7]. Metastasis involves multiple and complicated biochemical steps and events which are as follows: detachment, migration, invasion, and adhesion[8]. Though cancer cells are daily released in blood circulation, less than 0.1% of tumor cells metastasize according to melanoma studies[9]. The metastatic properties are determined by the nature of primary seeding cancer. This will determine their further growth and response to the treatment[10].
A metastatic lesion consists of cells that acquire the ability to deceive a series of molecular obstacles which would otherwise pose a hurdle to their non-metastasized counterparts[11]. A multi-step cascade is involved in the process of metastasis, from the dissemination of the cells to the formation of a new tumor colony. This process includes a sequence of events referred to as 'the invasion-metastasis cascade’. This sequence involves the following steps in general; the primary tumor cells invade local surrounding tissues, and local invasion of primary tumor cells into surrounding tissues followed by intravasation into the circulatory system. The cells need to survive this hematogenous transit. These cells eventually undergo extravasation into the parenchyma of the distant tissues, where they form multiple micro-metastatic colonies. This leads to colonization, where microscopic colonies proliferate into unconcealed and clinically detectable metastatic lesions[12].
The invasive potential of cells is determined by different genes, while specific mutations can also promote metastasis and invasion[13,14]. The genes that are predominantly involved in metastasis include cyclin-dependent kinase inhibitor 2A (CDKN2A), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), phosphatase and tensin homolog (PTEN), retinoblastoma (RB1) and tumor protein p53 (TP53)[15,16]. Tumor invasion and metastasis activation can be triggered by multiple epigenetic factors. These factors are induced by different stimuli which include circadian misalignment, and aging. Mechanical pressures, such as compression and tension in the extracellular matrix (ECM) and adhesive signaling molecules released from ECM components like collagen and fibrin also trigger metastasis. Other epigenetic factors comprise the intra-tumoral microbiome, cell-cell interactions, and growth factors[17,18].
One of the most important steps during the establishment of metastases is the formation of PMN. Primary cancer cells undergo a transition into an unregulated proliferative state which results in the development of secondary tumors once they colonize into a distant organ. The most frequent metastatic sites include organs such as bone, lungs, and liver.
PMN formation is a process that involves secretory factors, extracellular vesicles, secretory factors that lead to alteration of ECM, extravasation, and tumor-induced angiogenesis. Normal cells accept the vesicles carrying messenger RNA (mRNA) which are transcribed by metastasis-associated genes released by cancer cells. These genes allow the host cells to express a malignant phenotype, migrate and invade to distant organs[19–21]. Development of PMN involves an interaction of the primary tumor with the environment of organs. Once the cancer cells seed from the secondary organ, they interact with their environment creating the metastatic niche, stromal cells, immune cells, ECM, and the nutrient environment of the organ[22,23]. Thus, the formation of PMN is a crucial step in the process of cancer invasion and metastatic cascade by understanding and targeting these interacting sites that are involved in the development of PMN, thus providing an opportunity to prevent metastases before it is evinced[24].
EMT is a cellular process which is observed during embryogenesis and in the healing of epithelial tissues damage in adults. Cancer cells utilize this process for their benefit by equipping themselves with certain malignant traits, where the epithelial features are lost and are substituted with mesenchymal properties. Normal healthy epithelial cells are anchorage-dependent and are attached to the Extracellular matrix (ECM). Whereas, Metastatic cells survive by being detached from ECM. The neoplastic properties that are essential for metastatic dissemination and invasion, include the invasiveness and degradability of extracellular matrix (ECM) components. During this process, epithelial cells are transformed from organized and well differentiated stage to undifferentiated mesenchymal cells with migratory properties[25-28].
The TME comprises stromal components which include ECM, signaling factors, other macromolecules, and cellular elements that surround the tumor. These components and other elements may enter the bloodstream promoting local tumor growth and metastasis. Increased permeability of tumor microvasculature and cell migration can facilitate the leakage of these tumor elements and cellular components containing genetic material from the TME into the bloodstream. Thus, these elements in the bloodstream can be sampled and taken as a liquid biopsy for tumor analysis and profiling[5,29].
Liquid biopsies (LBs) are minimally invasive methods that are regularly and widely utilized in the surveillance, diagnosis, and management of solid and liquid tumors. Wide arrays of body fluids can be employed in LBs that are based on the type of cancer that needs to be studied[30]. Genomics, proteomics, and metabolomics are the most common omics techniques applied for the assessment of LB elements including, circulating tumor DNA (ctDNA), cell-free DNA (cfDNA), circulating tumor cells (CTCs), miRNA and extracellular vesicles (EVs)[31] (Fig. 2).
Blood samples are routinely used in LBs and are applicable for a broad range of cancers because blood is the only body fluid that is in contact with different parts of the body. Blood samples are easy to collect, store, transport, and analyze. It contains a significant amount of ctDNA, CTCs, circulatory miRNA, and EVs which can be analyzed using NGS, Mass spectrometry, and Polymerase Chain Reaction (PCR)[32]. For patients with advanced non-small cell lung cancer (NSCLC), molecular profiling of the EGFR T790M resistance mutation is essential for deciding the treatment strategy. This detection of mutation in ctDNA can be done by real-time allele-specific PCR and Sanger sequencing[33,34]. In an observational study involving ctDNA as an analyte in the blood samples of breast cancer patients, by using the targeted sequencing method for detection, it was found that the detection rate of ctDNA in pre-treated breast cancer was 67% (Stage I), 59% (Stage II) and 46% (Stage III)[35]. Circulating free microRNA from blood can be targeted for early diagnosis of breast cancer. Over expression of miR-125b-5p, miR-21–5p, miR-505–5p, and miR-96–5p was reported in a breast cancer patient before treatment (before any curative practice such as surgery, radiation, or systemic therapy) and decreased expression of miR-21–5p miR-3656, and miR-505–5p, was observed after the treatment The detection is carried out by using microarray and qRT-PCR[36]. The monitoring of circulating tumor cells (CTCs), enables early cancer detection and treatment effectiveness in different cancers. In the baseline CTCs count in the CTCs screening test, half of the subjects (n = 133 out of 265) had a count ranging from 0.2 to 50 CTC/mL (mean = 16 CTC/mL). About 20% (n = 24 out of 133) of screened people with a positive CTC count were observed with primary malignant lesions as confirmed by conventual imaging[37].
Urine biopsies are routinely employed in the diagnosis and surveillance of urological cancers due to the possibility that numerous urological cancer-related substances will flow directly into the urinary tract and to a smaller extent other cancer types. Castration-resistant prostate cancer (CRPC) is associated with Androgen-receptor splice variant 7 (AR-V7). The urinary EVs contain mRNA of AR-V7 which can be considered as the biomarker for CRPC and it is sensitively detected and quantified by using droplet digital PCR (ddPCR). Additionally, patients with CRPC had higher levels of AR-V7 expression and, more importantly, a larger AR-V7/AR-FL ratio than those with HSPC (hormone-sensitive prostate cancer)[38]. The metabolic studies of 25 endometrial cancer (EC) patients, Endometrial hyperplasia (EH) patients, and 25 healthy individuals were carried out by using Ultra-performance liquid chromatography (LC) quadrupole time of flight mass spectrometry (Q-TOF-MS). The data revealed that acetylcysteine and porphobilinogen were downregulated whereas Isobutyrylglycine, N-acetylserine, and urocanic acid were upregulated in EC individuals. The accuracy of this diagnostic model was 82.29% and new methods are being developed on metabolomics and proteomics platforms for discovering novel biomarkers in urine biopsies[39]. Long non-coding RNAs (lncRNAs) are potential biomarkers of carcinogenesis[40] and altered levels of various lncRNAs found in urinogenital malignancies which can be isolated and studied easily from urine samples. The levels of Prostate cancer antigen 3 (PCA3) (lncRNAs) was found to be elevated in more than 95% of prostate cancer case[41]. Furthermore, point of care (POC) devices can be applied to urine samples including lateral flow assays (LFA) to detect the protein biomarkers for various urological cancers from urine samples. Wang et.al 2008 have shown that in bladder cancer cells, NMP22 (Nuclear Metrix Protein 22) is overexpressed and can be detected in exfoliated bladder cancer cells (EBCCs) present in urine The US FDA-approved Bladderchek POC assay which can detect high concentrations of NMP22 protein (concentrations above 10 U/mL). It has further shown that a sensitivity and specificity of this assay by systematic review and quantitative meta-analysis is 56% (52–59%) and 88% (87–89%), respectively[42]. Due to the clear and less complex nature of urine, it can be applied as a POC device for rapid and accurate diagnosis of urological malignancies.
Saliva is an ideal candidate for developing new approaches for the management, diagnosis, and prognosis of oral cancers. Due to its ease of collection and larger sample quantities, a wealth of information can be obtained for studying disease progression and treatment response[43]. In a comparative study of 8 head and neck cancers, patients’ blood and saliva were analysed for five genes i.e., MDM2, CDKN1A, BBC3, DDB2, and BAX before and after radiotherapy using microarray analysis, quantitative PCR, and deep sequencing. The expression of these 5 genes remained the same in the blood samples. However, in salivary CDKN1A and DDB2 expressions were found to be significantly higher, and the degree of upregulation was associated with the dosage of radiotherapy[44]. Many miRNAs are discovered as potential biomarkers in salivary biopsies. One such study includes 22 nasopharyngeal carcinomas (NPC) patients and 25 healthy controls showed significant downregulation of 12 salivary miRNAs i.e., miR-3612, miR-4478, miR-937-5p, miR-650, miR-4259, miR-3714, miR-4730, miR-1203, miR-30b-3p, miR-1321, miR-1202, and miR575) which was validated with quantitative real-time-PCR. This model showed high accuracy with 100.00%, sensitivity, 0.999, Area under the curve [AUC] and 96.00% specificity proving that salivary miRNAs are promising biomarkers in oral cancers[45].
Bronchoalveolar lavage (BAL) is a kind of liquid biopsy used for lung cancer. It is obtained from a fibreoptic bronchoscopy procedure and it consider as minimally invasive process[46]. Methylation studies of RASSF1A and SHOX2 genes in BAL were performed on 130 healthy controls and 123 lung cancer cases to study the correlation between the lung cancer with RASSF1A and SHOX2 methylation levels. SHOX2 and RASSF1A had higher methylation-positive rates in the lung cancer group (64.2% and 50.4%, respectively) than in the control group (7.7% and 3.8%). The diagnostic efficiency of these two genes was high and has a specificity of 90.0% and sensitivity of 71.5%, compared to cytological examinations with sensitivity and specificity of 99.2% and 5.7%, respectively[47]. Label-free mass spectrometry-based proteomics approaches are used for the discovery of biomarkers from the BAL. The total sample size was 68 (controls = 16, NSCLC = 26, adenocarcinomas = 13, squamous cell carcinoma of the lung = 13) and it was reported that levels of four proteins (TIMP-1, Lipocalin-2, Cystatin-C, and HSP70/HSPA1) were found to be elevated in BAL of lung cancer patients[48]. To differentiate between non-cancerous patients (benign) suffering from lung fibrosis, Chronic Obstructive Pulmonary Disease (COPD), or asthma with lung cancer patients. The comparative study was conducted on 30 sample sizes, which lead to the identification of eight miRNAs (hsa-miR 1285, 1289, 1303, 217, 19b-1, 29a-5p, 548-3p, 650) using miRNA arrays. These results have shown differential expressions between the lung cancer group and the non-cancerous group. Five miRNAs such as U6 snRNA, hsa-miR 1303, 29a-5p, 1285, and 650, were considerably up-regulated in lung cancer patients, validated by qPCR[49].
The malignancies associated with the central nervous system (CNS) are very difficult to diagnose and monitor. Therefore, it is challenging to identify the grade of the tumor and the degree of metastasis in brain cancers. The tissue biopsies from brain tumors have a significantly higher risk of complications than most other tumors, which leads to information void about the tumor progression and this affects the treatment strategies[50]. Cerebrospinal fluid (CSF) is considered as the best liquid biopsy containing biomarkers for diagnosing the malignancies associated with CNS. Leptomeningeal metastases (LM) is a condition when a tumor spread through the inner membrane lining of the brain and spinal cord and thus the diagnosis of LM relies on CSF sampling[51]. The study conducted by Darlix et al. on CSF samples of 49 LM patients for analysing the presence of CTCs shows that the 18 patients had positive cytology results. CTCs were observed in 18 individuals, as well as in 5 patients with negative cytology. Clinical sensitivity for LM diagnosis was 100% and specificity was 77.3%. In the CSF sample of 40.6% of patients with HER2 breast cancer (BC), the Human epidermal growth factor receptor (HER2) + CTCs were found and the analysis was done on the CellSearch® system[52]. The presence of ctDNA in CSF is very well characterized in different CNS cancers[53]. The study was carried out on 38 telomerase reverse transcriptase (TERT) mutant glioblastoma samples (34 primary and 4 recurrent glioblastomas). ctDNA was sequenced unidirectionally on an Ion Torrent PGM NGS system, and the quantification cfDNA carried out on digital droplet PCR (ddPCR). These investigators have shown the importance of CSF-ctDNA for the precise and reliable detection of telomerase reverse transcriptase protein (TERTp) mutations in glioblastoma samples. These findings imply that high TERTp mutation in variant allele frequency (VAF) levels in the CSF-ctDNA may serve as a reliable indicator of poor survival in glioblastoma patients[54].
The cellular and molecular characterization of pleural fluid can give valuable insights into cancer management and monitoring. It is extensively studied in lung and breast cancers[55,56]. Pleural effusion (PE) is a condition of abnormal fluid accumulation between the pleura lining of the lung and the wall of the chest cavity. PE is mostly associated with lung and breast malignancies and is found to have significant levels of ctDNA, cfDNA/RNA, CTCs, EVs, and exosomes which can aid in molecular diagnosis of thoracic cancers[57,58]. In the past two decades, genomics characterization of PE has identified various mutations associated with lung and breast cancer. A study was conducted on 77 PFs and cell blocks, on 77 non-small cell lung cancer patients and reported mutations in EGFR gene in exon 20, at T790M, E20 insertion, exon 18 at G719X, exon 19 del, and exon 21 at L858R mutations. The RNA was extracted by PF sample and analysed by RTqPCR followed by DNA sequencing. The multiplex RT-PCR method is suitable for screening RET (protooncogene) rearrangements in NSCLC[59]. A similar study on exosomal RNA profiling was done and the exosomes were isolated from 36 plural effusion (PE) samples from 18 adenocarcinoma patients and 18 patients with benign inflammatory processes. The result showed a differential expression of 71 mRNAs and 17 miRNAs. It was found that the miR-200 family miRNAs and Lipocalin-2 (LCN2) were top differential expressed miRNA and mRNA transcripts respectively, proving their potential as a diagnostic biomarker in the PEs of the lung adenocarcinoma patients[60].
The tissue biopsy is known as a general cancer diagnostic method, and it can be use for genotyping, which can support targeted therapies. However, due to tumor heterogeneity and evolution, tissue biopsy-based cancer diagnostic methods have some restrictions in evaluating tumor growth, prognosis, and genotyping. The ctDNA is known to identify tumor-specific deformities, therefore, it is used as a biomarker for diagnosis and cancer therapeutic monitoring in liquid biopsies. The role of molecular profiling of cell-free DNA (cfDNA) in cancer therapy and diagnosis has recently been widely studied. The ctDNA exists in either single-stranded or double-stranded form in plasma or serum the presence of ctDNA is acknowledged but the exact origin of ctDNA remains unknown. It has been known that there are three possible ways of ctDNA origin 1) release through the breakdown of a cell through the process of apoptosis, necrosis, phagocytosis, and oncosis 2) active DNA release mechanism, and third is through the circulatory tumor. In cancer patients, three different sources of ctDNA are found either released by healthy cells, malignant cells, and TME cells[4, 61-63]. In 1948, Mandel and Metais revealed from tumors that ctDNA is part of cfDNA[64]. In 1977, Leon et al. found that cancer patients had more cfDNA than non-cancer patients[65]. It has been found that the amount of circulatory DNA increases with increasing tumor cells. The concentration of cfDNA in healthy patients ranges between 0–100 ng/mL but the same in cancer patients found that more than 1000 ng/mL[4,66]. Numerous cancer-related molecular factors have been found in ctDNA, including point mutations, methylation changes, and cancer-derived viral sequences[67].
The cfDNA is highly fragmented in which the ctDNA accounts for 0.01% of the whole cfDNA and the half-life of ctDNA is < 2.5 hours. These relatively low concentrations make their detection difficult, especially in the early stages of the tumor[68,69]. There are three different methods involved in cfDNA isolation. Phase isolation, silicon-membrane-based spin column, and magnetic beads isolation. Phase separation enables to achieve high isolation yield of cfDNA; on the other hand, cfDNA purification using magnetic bead isolation enables the recovery of more cfDNA compared to the silica membrane method. Although both spin columns and magnetic beads exhibit preferential retrieval for DNA fragments of a specific size. Markus et al., tested seven markedly available cfDNA extraction kits and observed that the QIAamp Circulating Nucleic Acid kit (Thermo Fisher) gave the maximum cfDNA yield and low-molecular-weight fractions. Alternative approaches which use the magnetic beads method for DNA extraction could be more automatable, MagMAX cfDNA extraction kit (Thermo Fisher) gives the maximum yield and low - molecular weight fractions[70,71]. Furthermore, altering the methylation status of ctDNA has been suggested as an additional biomarker to study therapeutic response[72]. The study conducted by Reece et.al 2019[73] have shown that ctDNA is useful in measuring the efficacy of surgical tumor clearance, and shifts in ctDNA level act as an indicator of the response to systemic treatments. It is possible to use ctDNA in targeted treatment. The emergence of new mutations and the reccurrence or elevated ctDNA correspond with tumor recurrence, development, and treatment resistance, the ctDNA quantification enabling more sensitive monitoring than presently used diagnostic tools.
The ctDNA levels depend on the cancer type. The tumor associated with the central nervous system releases the least amount of ctDNA in the blood because of the blood-brain barrier. The patients who have glioma there is a 90% chance that they do not show any detectable level of ctDNA[74-77]. Xu et al. developed a diagnostic prediction model using cfDNA samples from a large group of 1,098 individuals with hepatocellular carcinoma and 835 normal subjects, which demonstrated high diagnostic sensitivity and specificity (P < 0.001). It was strongly linked to tumour load, therapeutic response, and phase [78]. Osumi et al. studied the feasibility of using ctDNA to monitor tumor evolution in colorectal cancer patients. EVs and ctDNA have important roles in anti-PD-1/PD-L1 immunotherapy[79]. The findings suggest that tracking the mutation load is critical for assessing the response to PD-1-antibody-based immunotherapy[80]. To identify mutation-driven resistance, ctDNA can also be used to monitor therapy efficacy. Early diagnosis of ESR1 mutations, which is due to endocrine therapy resistance, could help to improve the outcomes of therapy before switching to another treatment in case of metastatic breast cancer[81]. The ctDNA can also use as a biomarker because it has a key methylation pattern that can be used to identify the epigenetic dysregulation of genes. hypermethylation of the RASSF1A, FHIT, and APC promoters observe in plasma DNA act as an ideal biomarker for diagnosis of early-stage renal cancer, with a sensitivity of 56.8% and a specificity of 96.7%[82].
The three main components of liquid biopsy are CTCs, ctDNA, and exosomes. Compared to CTCs and ctDNA, exosomes play a significant role in the monitoring of malignancies from liquid biopsies, as exosomes are abundant in a biological fluid and easy to isolate as compared to CTC and ctDNA. The quantity of exosomes in the blood sample is (~109 particles/ml) while there are only few CTCs present in 1 ml of the blood sample[83]. Exosomes are small (<150 mm), membrane-bound heterogeneous subtypes of EVs formed by the endocytosis pathway[84]. Exosomes are secreted by most the eukaryotic cell, and their function is to establish cell-cell communication apart from this they also act as transport vehicles to transport biomolecules like nucleic acid, protein, lipids, and other metabolites[85]. The exosomes that emerge from cells carry similar characteristics to their parent cells therefore, they can consider as a biomarker for disease diagnosis[86]. The DNA, mRNA, miRNA, long noncoding RNA, and cell-free circulating RNA (cfRNA) are constituents of exosomes, and they have a significant role in monitoring tumor progression, metastasis, and angiogenesis during cancer progression therefore, it used as a predictive biomarker to evaluate the severity of tumor in patients[87]. Like normal eukaryotic cells, the exosomes are also secreted by tumor cells. Exosomes secreted by tumor cells have specific biological functions such as facilitating the growth of cancer, involve in metastasis, and can modulate the immune system[88]. Exosomes are appealing biological markers of cancer, therapy resistance, and treatment response because they are sensitive and specific. Exosomes can provide an overview of the whole tumor as they can origin from any type of cell in the tumor cells[89,90]. The tumor creates TME, which is dominated by tumor-induced interactions. The extracellular matrix, stromal cells, and immune cells are constituted of TME[91]. Exosomes play a crucial role in TME by acting as efficient signaling molecules among cancerous cells and the surrounding TME cells. Exosome content in the endothelial cell may be useful as a marker for anti-angiogenic therapy potency, monitoring tumor cells, and representing transient cellular stress conditions[92].
The study conducted by Nakano et al. 2019[93] have shown the comparison between the level of expression of the exosomal miR92b gene and circulating alpha-fetoprotein (AFP) in hepatocellular carcinoma (HCC) patients who had received liver transplantation. They have concluded that exosomal miR-92b is more sensitive to predicting early HCC recurrence compared to AFP. The circulating exosome glypican 1 (GPC 1) is used as a potential biomarker in pancreatic ductal adenocarcinoma because of its high accuracy and specificity. Furthermore, the thresholds of circulating glypican before and after surgery have been correlated with tumor burden suggesting that GPC-1 has the potential and a reliable biomarker for both therapeutic and diagnostic monitoring[94]. The cancer cells are known to suppress the host immune response the one strategy is an increased expression of programmed death ligand (PD-L1), which binds PD-1 antigen-specific CD8 T-cells. In clinical research, it is found that exosomal PD-L1 is a possible indicator of response to anti-PD-1 therapy in an individual with melanoma and non-small cell lung cancer (NSCLC)[95,96]. The prostate-specific antigen (PSA) is commonly employed as a biomarker in prostate cancer. PSA is unable to differentiate between benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Exosomes are nanovesicles, that have been directly detectable in plasma samples of patients. As a result, the prospective use of plasmatic exosomes expressing PSA (Exo-PSA) can be used to differentiate between healthy people, BPH, and PCa[97]. According to Lin et al. 2018[98] have shown that the exosomal ephrin A2 derived from serum of prostate cancer patient is a good biomarker to distinguish between prostate cancer and BPH patients. Due to the significant importance of exosomes in liquid biopsy, exosome-based liquid biopsy has been evaluated in clinical trials, and few of them are accepted and are available in the market. ExoDxTM Lung (ALK), the world's first exosome-based liquid biopsy, was introduced in 2016 by Exosome Diagnostics for the separation and assessment of exosome RNA from blood samples[83]. Apart from the biomarker and early diagnosis, exosome protein has an important role in precision medicine and post-treatment disease monitoring.
Invasiveness and metastasis are two characteristic features of cancer cells. Metastasis is a multifaceted procedure in which cancer cells are released into the peripheral blood circulation in this mechanism they invade normal body cells. The CTCs were first discovered by Dr. Thomas Ashworth in 1869 where he described that "a few cells" from the blood of a metastatic cancer patient were found identical to primary tumor cells[99]. CTCs are the cells that are derived from primary tumor cells and transported into the peripheral blood circulation system. Therefore, the detection of CTCs in clinical specimens could serve as a prominent strategy to study cancer prognosis, metastasis, and diagnosis through liquid biopsy[100]. While being transported into the bloodstream, only a small percentage of CTCs get to survive. They are capable of forming a stable interaction with platelets, neutrophils, and macrophages to escape immune system surveillance[99]. Most of them are killed by either shear stress or through the process of anoikis. CTC concentrations in blood are relatively low[83], so a high throughput detection accuracy system and methods are necessary to identify such low concentrations in blood. A CTC screening system must have the ability to identify and differentiate diverse CTCs of all types, from whole blood and white blood cells[101]. Several recognition methods are available for the recognition of CTCs which include in-vitro detection methods, detection based on their physical and immunological property, size-dependent microfiltration, and biomarker-mediated detection methods. In-vitro detection methods include flow cytometry, magnetic beads, microfluidic chip, magnetic resonance imaging (MRI), positron emission tomography (PET), optical coherence tomography (OCT), and CT scan. A highly sensitive surface-charged superparamagnetic nanoprobe is currently a developed technique used for the extraction of CTCs. The character traits of CTCs can be used to recognize the tumor type. CTC recognition methodology is widely utilized in the early diagnosis and treatment of a diverse range of tumors, such as prostate cancer, digestive tract cancer, small cell lung cancer, and breast cancer[102]. Apart from the biomarker and early diagnosis CTCs also play a significant role in cancer treatment. Human epidermal growth factor (HER2 +) CTCs are observed in a subset of HER2- breast cancer patients[103].
A study conducted by Romiti et al. has shown the role of CTCs in cancer therapeutic monitoring by emphasizing the significance of CTC count before and after chemotherapy in patients with colorectal cancer. Sequencing of CTCs is also an important strategy in CTCs monitoring, for example in patients whose primary tumors are KRAS wild type in which the KRAS mutated CTCs are observed. This is revealed by ultra-deep sequence analysis, which would be impossible to reveal with a single biopsy[104]. The Cell SearchTM platform (Veridex LLC, USA) has become the most used cytometric CTC technology currently under clinical testing. It is the only technique to have obtained FDA approval for the estimation of CTCs in whole blood of a patient with metastatic breast, prostate, colorectal cancer[105]. However, this technique is unable to identify nonepithelial CTCs, such as epithelial-mesenchymal transition (EMT) + CTCs, resulting in a missed detection[106]. The monitoring of CTCs levels has an important role in the monitoring and evaluating of the disease condition, especially after chemotherapy and surgery. The real-time identification of CTCs levels in patients is required to track and assess their conditions, as well as detect and evaluate survival period[107].
Liquid biopsy is considered the most valuable and promising method in the area of cancer diagnosis. CTCs, ctDNA, exosomes, and TEP are considered a marker for liquid biopsy. Platelets and tumor cells interact in a variety of ways, and they are known to promote tumor growth and metastasis. Tumor-educated platelets (TEP) are platelets altered by the tumor cells, usually, tumor cells can alter the RNA pattern of platelets. So far, the available data demonstrated that the mRNA (transcriptome level analysis) in TEP can be employed for early diagnosis of cancer, with various potential applications[108,109]. It is known that TEPs play a significant role in the growth and metastasis of cancer. The altered RNA profile in TEPs is used as a biological marker for early diagnosis. It also provides precise information about tumor biology and molecular characteristics. While the diagnosis of TEP specimens from patients with various tumor types, such as lung, brain, and breast cancers, it has been proved that the TEPs pattern of cancer patients varies from those from inflammatory disease and other noncancerous diseases[110]. The platelets can carry protein derived from tumor cells. Therefore, the study of the alteration profile of protein content in platelets can be used for the early detection of cancer. The study conducted by Peterson 2012[111] has shown that the vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGE), and platelet factor 4 levels are more in plasma of colorectal cancer patients compared to the control. It is well known that transcriptomics level analysis of platelet RNA gives more insight into tumor pathology by taking this concept ahead Best et al, designed a Thromboseq, a transcriptome-based tool that can identify the RNA profile of platelets just from a single drop of blood. The tool has 96% diagnostic accuracy and 71% accuracy to determine the location of the tumor. This study was carried out on 228 individuals with different types of cancer including glioblastoma, NSCLC, colorectal, pancreatic, hepatobiliary, and breast cancer[112]. The Renal Cell Carcinoma (RCC) based pan-cancer TEPs model was created by Xiao et.al 2022[109] for early diagnosis and to investigate the clinical utility of TEPs in RCC patients. The study was conducted on 24 RCC patients and 25 healthy individuals. Platelet samples were collected for transcriptomic analysis to distinguish the differences in gene expression between RCC patients and controls.
Apart from their role as a biomarker and early diagnosis tool, platelet RNA does have the ability to integrate and make inferences from therapeutic strategies in addition to being a preliminary diagnostic tool. TEP RNA sampling for EML4-ALK mutations can indicate both positive and negative consequences in ALK1 NSCLC patients who received alectinib or crizotinib[113]. Furthermore, the overexpression of platelet-to-lymphocyte proportions before the treatment is linked with the lower response rate to nivolumab anti-PDL1 immunotherapy, suggesting that circulatory platelets could strengthen a pro-tumorigenic impact in the availability of an antitumor immune response[114,115].
miRNAs are small non-coding RNAs which control the expression of genes. They are involved in a variety of biological processes that occur under either normal or abnormal conditions, such as development, metabolic activity, tumor growth, metastasis, and immune response[116]. miRNA can be released by two types of mechanisms. First it can govern mRNA expression by entering a RNA-inducing silencing complex (RISC) or it can be directly secreted into biological fluids. Circulatory miRNAs can be classified into two types: vesicle-associated (which includes microvesicle exosomes or apoptotic bodies), and non-vesicle-associated (which includes cell-free miRNAs)[117]. The expression characterization of miRNA has been linked to tumorigenesis, and treatment response, implying that they could be used as diagnostic, prognostic, and predictive biological markers. Investigators used miRNA as a biomarker tool to examine the miRNA expression status of head and neck squamous cell carcinoma (HNSCC) and neighbouring normal tissues. They discovered that 6 miRNAs (miR-21, miR-1, miR-133a, miR-205, miR-206, and let-7d) had low activity levels when comparing to normal specimens[118]. The role of miRNA as a diagnostic biomarker is well known, the relationship between the expression of miRNA and their response in targeted therapy has also proposed their potential application to assist cancer therapeutics. Predicting a response to a particular therapy is clinically much more important. Lower levels of miR-26 are an impartial predictor in hepatocellular carcinoma (HCC); however, those who had low miR-26 did respond well to interferon treatment, which results in better survival. Therefore, it concludes that the miR-26 act as a biomarker to identify the patient who response very well to interferon-alpha therapy[119]. The abnormal level of miRNA has been seen in various phases of cancer. Therefore, it can be used as a useful clinical biomarker for tumor differentiation, to determine treatment strategy and monitor the treatment response. A group of miRNAs found in non-small cell lung cancer (NSCLC) patients which express differentially at various stages of cancer[120].
The main hurdle in cancer treatment is chemoresistance this is due to tumor heterogeneity; a few kinds of tumor cells could develop resistance upon prolonged therapy. Gemcitabine is a chemotherapy medication generally used for the treatment of pancreatic ductal adenocarcinoma (PDAC). It was found that prolonged administration of gemcitabine develops chemoresistance and it was associated with increased expression of miR-155-5p which stimulates gemcitabine resistance via anti-apoptotic action. Therefore, the expression profile of miR-155-5p can be used for therapeutic monitoring in PDAC patients who received gemcitabine therapy[121]. The overexpression of miR-125b indicates a lower response to Taxol-based therapies. However, the same finding was observed in the overexpression of miR-21 in pancreatic cancer patients who are undergoing treatment with gemcitabine[122,123]. Similarly, the lower expression of miR-146-5P concentration in serum exosomes is associated with cisplatin resistance[124]. The overexpression of miR-127-3p, miR-92a, and miR-486-3p and lower expression of miR-378 were linked with the occurrence of the KRAS mutation in colorectal cancer. Therefore, the miRNA expression profile can identify colorectal cancers that show resistance to EGF antagonists[125]. These putative-resistant miRNAs could be useful in assessing treatment resistance and acceptance, as well as selecting a clinical treatment strategy. Based on these findings, these miRNAs and their targets could be a unique approach to studying chemoresistance in metastatic cancer. In addition, you can the list of biological fluids and biomarkers used in liquid biopsies for diagnosis in Table 1.
| No. | Liquid Biopsies | Analyte | Application | Analytical Techniques | Cancer Diagnosed | References |
| ctDNA | Treatment strategy and responsiveness, Stratification of stages Diagnosis, | Real-time allele-specific PCR, sanger sequencing, TEC seq, NGS, whole-genome sequencing (WGS), ddPCR, whole-exome sequencing (WES) | NSCLC, Colorectal cancer, Breast cancer, Lung cancer | [33–35, 126,127] | ||
| 1 | Blood | miRNA | Diagnosis, prognosis, risk stratification | Microarray, qRT-PCR, ddPCR, NGS | Breast cancer, lung cancer, HNSCC, Prostate cancer | [36,128–130] |
| CTCs | Detection, treatment effectiveness, tumor relapse, drug resistance | Isolation-by-Size-of epithelial-Tumor (ISET) Methodology, Flow cytometry, CellSearch®, AdnaTest®, fluorescence in-situ hybridization (FISH) | Medulloblastoma, Gastrointestinal Cancers, Breast cancer, Colorectal cancer, Lung cancer, Prostate cancer, skin cancer | [37,131,132] | ||
| Exosomes | Diagnosis, tumor diagnosis, stratification of stages, | Proteomics, Immunoblotting, ELISA, qRT-PCR | Melanoma, Lung cancer, hepatocellular carcinoma | [133–135] | ||
| Urinary EVs | Diagnosis, disease progression | ddPCR, microarray, qRT-PCR, western blotting | Prostate cancer, bladder cancer | [38,136] | ||
| 2 | Urine | Metabolites | Diagnosis | Ultra-High Performance Liquid Chromatography (UHPLC) Quadrupole Time of Flight Mass Spectrometry (TOF-MS), LCMS, LCMS/MS | Endometrial cancer, HNSCC, renal cell carcinoma | [39,137,138] |
| ctDNA | Diagnosis, prognosis | ddPCR, WES, WGS, multiplex RTPCR | Bladder cancer, ovarian cancer | [139,140] | ||
| Metabolites | Diagnosis | NMR, MS, GC, HPLC, Capillary electrophoresis time-of-flight mass spectrometry | Oral squamous cell carcinoma (OSCC), Lung cancer | [141,142] | ||
| 3 | Saliva | miRNA | Diagnosis | Reverse transcriptase real-time- PCR (RT-qPCR) | Nasopharyngeal carcinoma, Gastric cancer | [45,143] |
| ctDNA | Diagnosis | NGS, WES, multiplex PCR, | HNSCC | [144] | ||
| DNA | Diagnosis | Sanger sequencing, qPCR, ddPCR | Lung cancer, NSCLC | [47,145] | ||
| 4 | BAL | Metabolites | Diagnosis | label-free mass spectrometry, direct infusion high-resolution mass spectrometry (DI-ESI-QTOF-MS), (GC-MS) | Lung cancer | [48,146] |
| CTCs | Diagnosis | FISH | Lung cancer | [147] | ||
| EVs | Diagnosis | qPCR | NSCLC | [148] | ||
| CTCs | Diagnosis, metastasis | CellSearch® system, Single-cell RNA sequencing | Leptomeningeal metastases (LM), lung cancer | [52,149] | ||
| 5 | CSF | ctDNA | Diagnosis, survival rate, characterization, monitoring | NGS, ddPCR, WES | Glioblastoma, medulloblastoma | [54,150] |
| miRNA | Disease progression and stratification, diagnosis | NGS qRT-PCR, microarrays | Medulloblastoma, Primary central nervous system lymphoma (PCNSL), Glioblastoma | [151,152] | ||
| ctDNA | Diagnosis | NGS, qRT-PCR, qPCR, | Lung cancer, lung adenocarcinoma | [153,154] | ||
| 6 | Pleural fluid | CTCs | Diagnosis, prognosis, mesenchymal-epithelial transition | Western blot arrays, cell search | Small cell lung cancer (SCLC), malignant mesothelioma | [155,156] |
| EVs | Diagnosis, prognosis | MicroRNA microarray, qRT-PCR | Esophageal cancer, ovarian cancer | [157,158] | ||
| The blood, saliva, urine, CSF, BAL, and pleural fluid are routinely used as a liquid biopsy and ctDNA, exosomes, CTCs, tumor-educated platelets (TEPs), and miRNA are used as targeted biomarkers. | ||||||
The most routinely available diagnostic method for cancer is tissue biopsy. The sample collected from the tissue biopsy is typically taken from the primary tumor and they represent the molecular profile of the tumor at the time when samples were taken. Therefore, there is a chance that some of the property can remain undetected. In contrast, in liquid biopsies, samples are directly taken from biological fluids which makes it possible to study the malignancy property of the tumor as well as real- time monitoring of treatment response. Liquid biopsy has started a new era in the field of early-stage cancer diagnostic and therapeutic monitoring. The biomarkers analysed in liquid biopsies can be used for real-time monitoring of cancer prognosis, development, and response to the therapy. Available studies have shown that liquid biopsies can be used to study different stages of metastatic cancer. Therefore, it is possible to modify treatment strategies based on the cancer stages and their response. This highlighted the role of liquid biopsies in precision medicines and use of a targeted therapy for better treatment of metastatic cancer. The biomarkers used for diagnosis and monitoring in liquid biopsies are mainly circulating tumor DNA, circulating tumor cells, miRNA, exosomes, and TEPs which are isolated from biological fluids such as blood, urine, cerebral spinal fluid, saliva, bronchoalveolar lavages are analysed by using molecular biology techniques such as NGS, molecular profiling etc. Therefore, the use of liquid biopsies in conjunction with otther diagnostic tests, such as imaging tests and tissue biopsies, can provide a clearer picture of cancer and help to guide treatment decisions. The role of liquid biopsies in the area of cancer diagnosis is a subject of great interest and importance, that can be used in the area of personalized cancer medicine.
AFP, Alpha-fetoprotein; AR-V7, Androgen-receptor splice variant 7; AUC, Area under the curve; BAL, Bronchoalveolar lavage; BPH, Benign prostatic hyperplasia; CDKN2A, Cyc-lin-dependent kinase inhibitor 2A; cfDNA, Cell free DNA; CNS, Central nervous system; COPD, Chronic Obstructive Pulmonary Disease; CRPC, Castration-resistant prostate cancer; Cell free DNA; CNS, Central nervous system; COPD, Chronic Obstructive Pulmonary Disease; CRPC, Castration-resistant prostate cancer; CSF, Cerebrospinal fluid; CTCs, Circulating tumor cells cell-free DNA; ddPCR, Droplet digital polymerase chain reaction; EBCCs, Exfoli-ated bladder cancer cells; EC, Endometrial cancer; ECM, Extracellular matrix; EGFR, epidermal growth factor receptor; EH, Endometrial hyper-plasia; EMT, Epithelial-mesenchymal transition; EVs, Extracellular vesicles; Exo-PSA, Exosome expressing prostate specific antigen; FISH, Florescence in-situ hybridization; GPC 1, Glypican 1; HCC, Hepatocellular carcinoma; HER, Human epidermal growth factor receptor; HNSCC, Head and neck squamous cell carcinomas; HPLC, High performance liquid chromatography; HSPC, Hormone specific prostate cancer; LBs, Liquid biopsies; LC, Liquid chromatography; LC-MS/MS, Liquid chromatography mass spectrometry; LCN2, Lipocalin-2; LFA, Lateral flow assay; LM, Leptomeningeal metastases; lncRNAs, long non coding RNAs; miRNA, MicroRNA; miRNA, MicroRNA; MRI, Magnetic resonance imaging; NGS, Next generation sequencing; NMP22, Nuclear matrix protein 22; nbsp;Hormone specific prostate cancer; LBs, Liquid biopsies; LC, Liquid chromatography; LC-MS/MS, Liquid chromatography mass spectrometry; LCN2, Lipocalin-2; LFA, Lateral flow assay; LM, Leptomeningeal metastases; lncRNAs, long non coding RNAs; miRNA, MicroRNA; miRNA, MicroRNA; MRI, Magnetic resonance imaging; NPC, Nasopharyngeal carcinomas; NSCLC, Non-small cell lung cancer; OCT, Optical coherence tomography; PCA, Prostate cancer antigen; PCa, Prostate cancer; PDAC, Pancreatic ductal adenocarcinoma; PDGE, Platelet derived growth factor; PDL1, Program death ligand; PE, Pleural effusion; PET, Positron emission tomography; PF, Pleural fluid; PC, Nasopharyngeal carcinomas; NSCLC, Non-small cell lung cancer; OCT, Optical coherence tomography; PCA, Prostate cancer antigen; PCa, Prostate cancer; PDAC, Pancreatic ductal adenocarcinoma; PDGE, Platelet derived growth factor; PDL1, Program death ligand; PE, Pleural effusion; PET, Positron emission tomography; PF, Pleural fluid; PMN, Pre-metastatic niche; POC, Point of care; PSA, Prostate specific antigen; PTEN, Phosphatase and tensinhomolog; qRT-PCR, Quantitative real time polymerase chain reaction; Q-TOF MS Quadrupole time of flight mass spectrometer; RB1, Retinoblastoma; RCC, Renal cell carcinoma; RISC, RNA-induced silencing complex; TEPs, Tumor educated platelets; MN, Pre-metastatic niche; POC, Point of care; PSA, Prostate specific antigen; PTEN, Phosphatase and tensinhomolog; qRT-PCR, Quantitative real time polymerase chain reaction; Q-TOF MS Quadrupole time of flight mass spectrometer; RB1, Retinoblastoma; TERT, Telomerase reverse transcript; TME, Tumor microenvironment; TP53, Tumor protein p53; UHPLC, Ultra high performance liquid chromatography; VEGF, Vascular endothelial growth factor.
OK contributed towards preparing the outline for the manuscript, editing, and formatting. OK, VP and SN contributed in the construction and writing of the manuscript. AC has the contribution for the proofreading of the manuscript. VP contributed in preparing the figures. SN contributed towards the preparation of all tables.
The authors declare that they have no conflicts of interest.
* Sagar Nagrekar and Vivek Parab are the second authorship.
| [1] |
Perakis S, Speicher MR. Emerging Concepts in Liquid Biopsies. BMC Med, 2017, 15(1): 1-12. DOI: 10.1186/s12916-016-0759-3
|
| [2] |
Siravegna G, Marsoni S, Siena S, et al. Integrating Liquid Biopsies into the Management of Cancer. Nat Rev Clin Oncol, 2017, 14(9): 531-548. DOI: 10.1038/nrclinonc.2017.14
|
| [3] |
Alix-Panabières C, Pantel K. Technologies for Detection of Circulating Tumor Cells: Facts and Vision. Lab Chip, 2014, 14(1): 57-62. DOI: 10.1039/C3LC50644D
|
| [4] |
Thierry AR, El Messaoudi S, Gahan PB, et al. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev, 2016, 35(3): 347-376. DOI: 10.1007/s10555-016-9629-x
|
| [5] |
Underwood JJ, Quadri RS, Kalva SP, et al. Liquid Biopsy for Cancer: Review and Implications for the Radiologist. Radiology, 2020, 294(1): 5-17. DOI: 10.1148/radiol.2019182584
|
| [6] |
Weber GF. Why Does Cancer Therapy Lack Effective Anti-Metastasis Drugs? Cancer Lett, 2013, 328(2): 207-211.
|
| [7] |
Lin Q, Lu L, Wang X, et al. Neoadjuvant chemotherapy plus intensity-modulated radiotherapy versus neoadjuvant chemotherapy plus concurrent chemoradiotherapy for ascending or descending types of nasopharyngeal carcinoma: A retrospective study. Am J Otolaryngol, 2022, 43(1): 103193. DOI: 10.1016/j.amjoto.2021.103193
|
| [8] |
Wells A, Grahovac J, Wheeler S, et al. Targeting Tumor Cell Motility as a Strategy against Invasion and Metastasis. Trends Pharmacol Sci, 2013, 34(5): 283-289. DOI: 10.1016/j.tips.2013.03.001
|
| [9] |
Luzzi KJ, MacDonald IC, Schmidt EE, et al. Multistep Nature of Metastatic Inefficiency Dormancy of Solitary Cells after Successful Extravasation and Limited Survival of Early Micrometastases. Am J Pathol, 1998, 153(3): 865-873. DOI: 10.1016/S0002-9440(10)65628-3
|
| [10] |
Tabassum DP, Polyak K. Tumorigenesis: It Takes a Village. Nat Rev Cancer, 2015, 15(8): 473-483. DOI: 10.1038/nrc3971
|
| [11] |
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell, 2017, 168(4): 670-691. DOI: 10.1016/j.cell.2016.11.037
|
| [12] |
Reymond N, d'Agua BB, Ridley AJ. Crossing the Endothelial Barrier during Metastasis. Nat Rev Cancer, 2013, 13(12): 858-870. DOI: 10.1038/nrc3628
|
| [13] |
Ramaswamy S, Ross KN, Lander ES, et al. A Molecular Signature of Metastasis in Primary Solid Tumors. Nat Genet, 2003, 33(1): 49-54. DOI: 10.1038/ng1060
|
| [14] |
Van't Veer LJ, Dai H, Van De Vijver MJ, et al. Gene Expression Profiling Predicts Clinical Outcome of Breast Cancer. Nature, 2002, 415(6871): 530-536. DOI: 10.1038/415530a
|
| [15] |
Robinson DR, Wu YM, Lonigro RJ, et al. Integrative Clinical Genomics of Metastatic Cancer. Nature, 2017, 548(7667): 297-303. DOI: 10.1038/nature23306
|
| [16] |
Birkbak NJ, McGranahan N. Cancer Genome Evolutionary Trajectories in Metastasis. Cancer Cell, 2020, 37(1): 8-19. DOI: 10.1016/j.ccell.2019.12.004
|
| [17] |
Kaur A, Ecker BL, Douglass SM, et al. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov, 2019, 9(1): 64-81. DOI: 10.1158/2159-8290.CD-18-0193
|
| [18] |
Profumo V, Gandellini P. MicroRNAs: Cobblestones on the Road to Cancer Metastasis. Crit Rev Oncog, 2013, 18(4): 341-355. DOI: 10.1615/CritRevOncog.2013007182
|
| [19] |
Papalazarou V, Zhang T, Paul NR, et al. The Creatine–Phosphagen System Is Mechanoresponsive in Pancreatic Adenocarcinoma and Fuels Invasion and Metastasis. Nat Metab, 2020, 2(1): 62-80. DOI: 10.1038/s42255-019-0159-z
|
| [20] |
Peinado H, Zhang H, Matei IR, et al. Pre-Metastatic Niches: Organ-Specific Homes for Metastases. Nat Rev Cancer, 2017, 17(5): 302-317. DOI: 10.1038/nrc.2017.6
|
| [21] |
Zomer A, Maynard C, Verweij FJ, et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior. Cell, 2015, 161(5): 1046-1057. DOI: 10.1016/j.cell.2015.04.042
|
| [22] |
Janssen LME, Ramsay EE, Logsdon CD, et al. The Immune System in Cancer Metastasis: Friend or Foe? J Immunother Cancer, 2017, 5: 1-14. DOI: 10.1186/s40425-016-0206-1
|
| [23] |
Elia I, Doglioni G, Fendt SM. Metabolic Hallmarks of Metastasis Formation. Trends Cell Biol, 2018, 28(8): 673-684. DOI: 10.1016/j.tcb.2018.04.002
|
| [24] |
Doglioni G, Parik S, Fendt SM. Interactions in the (Pre)Metastatic Niche Support Metastasis Formation. Front Oncol, 2019, 9: 219. DOI: 10.3389/fonc.2019.00219
|
| [25] |
Alizadeh AM, Shiri S, Farsinejad S. Metastasis Review: From Bench to Bedside. Tumor Biol, 2014, 35(9): 8483-8523. DOI: 10.1007/s13277-014-2421-z
|
| [26] |
Kalluri R, Weinberg RA. The Basics of Epithelial-Mesenchymal Transition. J Clin Invest, 2009, 119(6): 1420-1428. DOI: 10.1172/JCI39104
|
| [27] |
Thiery JP. Epithelial–Mesenchymal Transitions in Tumour Progression. Nat Rev Cancer, 2002, 2(6): 442-454. DOI: 10.1038/nrc822
|
| [28] |
Nieto MA, Huang RYJ, Jackson RA, et al. EMT: 2016. Cell, 2016, 166(1): 21-45. DOI: 10.1016/j.cell.2016.06.028
|
| [29] |
Spill F, Reynolds DS, Kamm RD, et al. Impact of the Physical Microenvironment on Tumor Progression and Metastasis. Curr Opin Biotechnol, 2016, 40: 41-48. DOI: 10.1016/j.copbio.2016.02.007
|
| [30] |
Martins I, Ribeiro IP, Jorge J, et al. Liquid biopsies: applications for cancer diagnosis and monitoring. Genes, 2021, 12(3): 349. DOI: 10.3390/genes12030349
|
| [31] |
Amelio I, Bertolo R, Bove P, et al. Liquid biopsies and cancer omics. Cell Death Discov, 2020, 6(1): 131. DOI: 10.1038/s41420-020-00373-0
|
| [32] |
Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci, 2018, 19(10): 2877. DOI: 10.3390/ijms19102877
|
| [33] |
Maguire T. ONtario Health Technology Assessment Series Cell-free Circulating Tumour DNA Blood Testing to Detect EGFR T790M Mutation in People with Advanced Non-Small Cell Lung Cancer: A Health Technology Assessment. Ont Health Technol Assess Ser. 2020;20(5):1–176.
|
| [34] |
Hor SY, Chan KS, Chen EX, et al. Detection of EGFR T790M resistance mutation: real-time allele-specific PCR versus Sanger sequencing. Ann Oncol, 2016, 27: vi431. DOI: 10.1093/annonc/mdw383.49
|
| [35] |
Alba-Bernal A, Lavado-Valenzuela R, Domínguez-Recio ME, et al. Challenges and achievements of liquid biopsy technologies employed in early breast cancer. EBioMedicine, 2020, 62: 103100. DOI: 10.1016/j.ebiom.2020.103100
|
| [36] |
Matamala N, Vargas MT, Gonzalez-Campora R, et al. Tumor microRNA expression profiling identifies circulating microRNAs for early breast cancer detection. Clin Chem, 2015, 61(8): 1098-1106. DOI: 10.1373/clinchem.2015.238691
|
| [37] |
Ried K, Eng P, Sali A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effectiveness: an observational study. Asian Pac J Cancer Prev, 2017, 18(8): 2275. DOI: 10.22034/APJCP.2017.18.8.2275
|
| [38] |
Woo HK, Park J, Ku JY, et al. Urine-based liquid biopsy: non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab on a Chip, 2019, 19(1): 87-97. DOI: 10.1039/C8LC01185K
|
| [39] |
Shao X, Wang K, Liu X, et al. Screening and verifying endometrial carcinoma diagnostic biomarkers based on a urine metabolomic profiling study using UPLC-Q-TOF/MS. Clin Chim Acta, 2016, 463: 200-206. DOI: 10.1016/j.cca.2016.10.027
|
| [40] |
Wang F, Li X, Xie XJ, et al. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett, 2008, 582(13): 1919-1927. DOI: 10.1016/j.febslet.2008.05.012
|
| [41] |
Bussemakers MJG, Van Bokhoven A, Verhaegh GW, et al. Dd3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res, 1999, 59(23): 5975-5979.
|
| [42] |
Moonen PMJ, Kiemeney L, Witjes JA. Urinary NMP22® BladderChek® test in the diagnosis of superficial bladder cancer. Eur Urol, 2005, 48(6): 951-956. DOI: 10.1016/j.eururo.2005.09.002
|
| [43] |
Arantes LMRB, De Carvalho AC, Melendez ME, et al. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev Mol Diagn, 2018, 18(1): 85-112. DOI: 10.1080/14737159.2017.1404906
|
| [44] |
Lacombe J, Brooks C, Hu C, et al. Analysis of saliva gene expression during head and neck cancer radiotherapy: a pilot study. Radiat Res, 2017, 188(1): 75-81. DOI: 10.1667/RR14707.1
|
| [45] |
Wu L, Zheng K, Yan C, et al. Genome-wide study of salivary microRNAs as potential noninvasive biomarkers for detection of nasopharyngeal carcinoma. BMC Cancer, 2019, 19(1): 1-11. DOI: 10.1186/s12885-018-5219-3
|
| [46] |
Alharbi KK. Clinical efficacy and possible applications of genomics in lung cancer. Asian Pac J Cancer Prev, 2015, 16(5): 1693-1698. DOI: 10.7314/APJCP.2015.16.5.1693
|
| [47] |
Ren M, Wang C, Sheng D, et al. Methylation analysis of SHOX2 and RASSF1A in bronchoalveolar lavage fluid for early lung cancer diagnosis. Ann Diagn Pathol, 2017, 27: 57-61. DOI: 10.1016/j.anndiagpath.2017.01.007
|
| [48] |
Hmmier A, O'Brien ME, Lynch V, et al. Proteomic analysis of bronchoalveolar lavage fluid (BALF) from lung cancer patients using label-free mass spectrometry. BBA Clin, 2017, 7: 97-104. DOI: 10.1016/j.bbacli.2017.03.001
|
| [49] |
Rehbein G, Schmidt B, Fleischhacker M. Extracellular microRNAs in bronchoalveolar lavage samples from patients with lung diseases as predictors for lung cancer. Clin Chim Acta, 2015, 450: 78-82. DOI: 10.1016/j.cca.2015.07.027
|
| [50] |
Omuro AMP, Leite CC, Mokhtari K, et al. Pitfalls in the diagnosis of brain tumours. Lancet Neurol, 2006, 5(11): 937-948. DOI: 10.1016/S1474-4422(06)70597-X
|
| [51] |
Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol, 2019, 135: 85-94. DOI: 10.1016/j.critrevonc.2019.01.020
|
| [52] |
Darlix A, Cayrefourcq L, Pouderoux S, et al. Detection of Circulating Tumor Cells in Cerebrospinal Fluid of Patients with Suspected Breast Cancer Leptomeningeal Metastases: A Prospective Study. Clin Chem, 2022, 68(10): 1311-1322. DOI: 10.1093/clinchem/hvac127
|
| [53] |
Escudero L, Martínez-Ricarte F, Seoane J. ctDNA-based liquid biopsy of cerebrospinal fluid in brain cancer. Cancers, 2021, 13(9): 1989. DOI: 10.3390/cancers13091989
|
| [54] |
Juratli TA, Stasik S, Zolal A, et al. TERT Promoter Mutation Detection in Cell-Free Tumor-Derived DNA in Patients with IDH Wild-Type Glioblastomas: A Pilot Prospective StudyTERT Promoter Mutations in Glioblastoma Cell-Free Tumor DNA. Clin Cancer Res, 2018, 24(21): 5282-5291. DOI: 10.1158/1078-0432.CCR-17-3717
|
| [55] |
Schwed Lustgarten DE, Thompson J, Yu G, et al. Use of circulating tumor cell technology (CELLSEARCH) for the diagnosis of malignant pleural effusions. Ann Am Thorac Soc, 2013, 10(6): 582-589. DOI: 10.1513/AnnalsATS.201303-068OC
|
| [56] |
Grigoriadou GΙ, Esagian SM, Ryu HS, et al. Molecular profiling of malignant pleural effusions with next generation sequencing (NGS): evidence that supports its role in cancer management. J Pers Med, 2020, 10(4): 206. DOI: 10.3390/jpm10040206
|
| [57] |
Sorolla MA, Sorolla A, Parisi E, et al. Diving into the pleural fluid: liquid biopsy for metastatic malignant pleural effusions. Cancers, 2021, 13(11): 2798. DOI: 10.3390/cancers13112798
|
| [58] |
Baburaj G, Damerla RR, Udupa KS, et al. Liquid biopsy approaches for pleural effusion in lung cancer patients. Mol Biol Rep, 2020, 47: 8179-8187. DOI: 10.1007/s11033-020-05869-7
|
| [59] |
Tsai TH, Wu SG, Hsieh MS, et al. Clinical and prognostic implications of RET rearrangements in metastatic lung adenocarcinoma patients with malignant pleural effusion. Lung Cancer, 2015, 88(2): 208-214. DOI: 10.1016/j.lungcan.2015.02.018
|
| [60] |
Hydbring P, De Petris L, Zhang Y, et al. Exosomal RNA-profiling of pleural effusions identifies adenocarcinoma patients through elevated miR-200 and LCN2 expression. Lung Cancer, 2018, 124: 45-52. DOI: 10.1016/j.lungcan.2018.07.018
|
| [61] |
Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget, 2016, 7(30): 48832. DOI: 10.18632/oncotarget.9453
|
| [62] |
Pessoa LS, Heringer M, Ferrer VP. ctDNA as a cancer biomarker: A broad overview. Crit Rev Oncol Hematol, 2020, 155: 103109. DOI: 10.1016/j.critrevonc.2020.103109
|
| [63] |
Aucamp J, Bronkhorst AJ, Badenhorst CPS, et al. The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol Rev, 2018, 93(3): 1649-1683. DOI: 10.1111/brv.12413
|
| [64] |
Schmidt B, Weickmann S, Witt C, et al. Improved method for isolating cell-free DNA. Clin Chem, 2005, 51(8): 1561-1563.
|
| [65] |
Sánchez-Herrero E, Serna-Blasco R, Robado de Lope L, et al. Circulating Tumor DNA as a Cancer Biomarker: An Overview of Biological Features and Factors That may Impact on ctDNA Analysis. Front Oncol, 2022: 12. DOI: 10.3389/fonc.2022.943253
|
| [66] |
Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer, 2011, 11(6): 426-437. DOI: 10.1038/nrc3066
|
| [67] |
Okajima W, Komatsu S, Ichikawa D, et al. Liquid biopsy in patients with hepatocellular carcinoma: circulating tumor cells and cell-free nucleic acids. World J Gastroenterol, 2017, 23(31): 5650. DOI: 10.3748/wjg.v23.i31.5650
|
| [68] |
Elazezy M, Joosse SA. Techniques of using circulating tumor DNA as a liquid biopsy component in cancer management. Comput Struct Biotechnol J, 2018, 16: 370-378. DOI: 10.1016/j.csbj.2018.10.002
|
| [69] |
Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res, 2004, 10(24): 8152-8162. DOI: 10.1158/1078-0432.CCR-04-1110
|
| [70] |
Markus H, Contente-Cuomo T, Farooq M, et al. Evaluation of pre-analytical factors affecting plasma DNA analysis. Sci Rep, 2018, 8(1): 7375. DOI: 10.1038/s41598-018-25810-0
|
| [71] |
Lu JL, Liang ZY. Circulating free DNA in the era of precision oncology: Pre-and post-analytical concerns. Chronic Dis Transl Med, 2016, 2(4): 223-230. DOI: 10.1016/j.cdtm.2016.12.001
|
| [72] |
Thomsen CB, Hansen TF, Andersen RF, et al. Early identification of treatment benefit by methylated circulating tumor DNA in metastatic colorectal cancer. Ther Adv Med Oncol, 2020, 12: 1758835920918472. DOI: 10.1177/1758835920918472
|
| [73] |
Reece M, Saluja H, Hollington P, et al. The use of circulating tumor DNA to monitor and predict response to treatment in colorectal cancer. Front Genet, 2019, 10: 1118. DOI: 10.3389/fgene.2019.01118
|
| [74] |
Jiang BY, Li YS, Guo WB, et al. Detection of Driver and Resistance Mutations in Leptomeningeal Metastases of NSCLC by Next-Generation Sequencing of Cerebrospinal Fluid Circulating Tumor CellsCSFCTCs in Leptomeningeal Metastases of NSCLC. Clin Cancer Res, 2017, 23(18): 5480-5488. DOI: 10.1158/1078-0432.CCR-17-0047
|
| [75] |
Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci Transl Med, 2014, 6(224): 224ra24-224ra24. DOI: 10.1126/scitranslmed.3007094
|
| [76] |
Pan W, Gu W, Nagpal S, et al. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem, 2015, 61(3): 514-522. DOI: 10.1373/clinchem.2014.235457
|
| [77] |
Huang Z, Gu B. Circulating Tumor DNA: A Resuscitative Gold Mine? Ann Transl Med, 2015, 3(17): 253. DOI: 10.3978/j.issn.2305-5839.2015.09.11
|
| [78] |
Xu R, Wei W, Krawczyk M, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater, 2017, 16(11): 1155-1162. DOI: 10.1038/nmat4997
|
| [79] |
Osumi H, Shinozaki E, Yamaguchi K, et al. Clinical utility of circulating tumor DNA for colorectal cancer. Cancer Sci, 2019, 110(4): 1148-1155. DOI: 10.1111/cas.13972
|
| [80] |
Huang C, Liu S, Tong X, et al. Extracellular vesicles and ctDNA in lung cancer: biomarker sources and therapeutic applications. Cancer Chemother Pharmacol, 2018, 82: 171-183. DOI: 10.1007/s00280-018-3586-8
|
| [81] |
Fribbens C, O'Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol, 2016, 34(25): 2961-2968. DOI: 10.1200/JCO.2016.67.3061
|
| [82] |
Skrypkina I, Tsyba L, Onyshchenko K, et al. Concentration and methylation of cell-free DNA from blood plasma as diagnostic markers of renal cancer. Dis Markers, 2016, 2016.
|
| [83] |
Yu D, Li Y, Wang M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer, 2022, 21(1): 56. DOI: 10.1186/s12943-022-01509-9
|
| [84] |
Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol, 2014, 29: 116-125. DOI: 10.1016/j.ceb.2014.05.004
|
| [85] |
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science, 2020, 367(6478): eaau6977. DOI: 10.1126/science.aau6977
|
| [86] |
Wang X, Tian L, Lu J, et al. Exosomes and cancer-Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis, 2022, 11(1): 54. DOI: 10.1038/s41389-022-00431-5
|
| [87] |
Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther, 2020, 5(1): 145. DOI: 10.1038/s41392-020-00261-0
|
| [88] |
Chanteloup G, Cordonnier M, Isambert N, et al. Monitoring HSP70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J Extracell Vesicles, 2020, 9(1): 1766192. DOI: 10.1080/20013078.2020.1766192
|
| [89] |
Wang J, De Veirman K, Faict S, et al. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol, 2016, 239(2): 162-173. DOI: 10.1002/path.4712
|
| [90] |
Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer, 2019, 18: 1-10. DOI: 10.1186/s12943-019-0975-5
|
| [91] |
Roma-Rodrigues C, Mendes R, Baptista PV, et al. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci, 2019, 20(4): 840. DOI: 10.3390/ijms20040840
|
| [92] |
de Jong OG, Verhaar MC, Chen Y, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles, 2012, 1(1): 18396. DOI: 10.3402/jev.v1i0.18396
|
| [93] |
Nakano T, Chen IH, Wang CC, et al. Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am J Transplant, 2019, 19(12): 3250-3262. DOI: 10.1111/ajt.15490
|
| [94] |
Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature, 2015, 523(7559): 177-182. DOI: 10.1038/nature14581
|
| [95] |
Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature, 2014, 515(7528): 568-571. DOI: 10.1038/nature13954
|
| [96] |
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J med, 2016, 375: 1823-1833. DOI: 10.1056/NEJMoa1606774
|
| [97] |
Logozzi M, Angelini DF, Giuliani A, et al. Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: a prospective study. Cancers, 2019, 11(10): 1449. DOI: 10.3390/cancers11101449
|
| [98] |
Li S, Zhao Y, Chen W, et al. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J Cancer, 2018, 9(15): 2659. DOI: 10.7150/jca.25201
|
| [99] |
Lin D, Shen L, Luo M, et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct Target Ther, 2021, 6(1): 404. DOI: 10.1038/s41392-021-00817-8
|
| [100] |
Deng Z, Wu S, Wang Y, et al. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine, 2022, 83:104237.
|
| [101] |
Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol, 2016, 10(3): 374-394. DOI: 10.1016/j.molonc.2016.01.007
|
| [102] |
Zhang H, Lin X, Huang Y, et al. Detection methods and clinical applications of circulating tumor cells in breast cancer. Front Oncol, 2021, 11: 652253. DOI: 10.3389/fonc.2021.652253
|
| [103] |
Lopes C, Piairo P, Chícharo A, et al. HER2 expression in circulating tumour cells isolated from metastatic breast cancer patients using a size-based microfluidic device. Cancers, 2021, 13(17): 4446. DOI: 10.3390/cancers13174446
|
| [104] |
Potdar PD, Lotey NK. Role of circulating tumor cells in future diagnosis and therapy of cancer. J Cancer Metastasis Treat, 2015, 1: 44-56. DOI: 10.4103/2394-4722.158803
|
| [105] |
Krebs MG, Hou JM, Ward TH, et al. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol, 2010, 2(6): 351-365. DOI: 10.1177/1758834010378414
|
| [106] |
Kalyani A, Jha RM, Sharma S. Use of circulating nucleic acids, metabolites, and proteins as clinical biomarkers for earlier prognosis and diagnosis of disease. Transl Res, 2019: 85-116.
|
| [107] |
Cai F, Cen C, Cai L, et al. Application of circulation tumor cells detection in diagnosis and treatment of breast tumors. Rejuvenation Res, 2019, 22(6): 498-502. DOI: 10.1089/rej.2018.2154
|
| [108] |
Varkey J, Nicolaides T. Tumor-educated platelets: A review of current and potential applications in solid tumors. Cureus, 2021, 13(11).
|
| [109] |
Xiao R, Liu C, Zhang B, et al. Tumor-educated platelets as a promising biomarker for blood-based detection of renal cell carcinoma. Front Oncol, 2022: 689.
|
| [110] |
Best MG, Wesseling P, Wurdinger T. Tumor-Educated Platelets as a Noninvasive Biomarker Source for Cancer Detection and Progression MonitoringTEPs for Blood-Based Cancer Diagnostics. Cancer Res, 2018, 78(13): 3407-3412. DOI: 10.1158/0008-5472.CAN-18-0887
|
| [111] |
Peterson JE, Zurakowski D, Italiano JE, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis, 2012, 15: 265-273. DOI: 10.1007/s10456-012-9259-z
|
| [112] |
Roweth HG, Battinelli EM. Lessons to learn from tumor-educated platelets. Blood, 2021, 137(23): 3174-3180. DOI: 10.1182/blood.2019003976
|
| [113] |
Park CK, Kim JE, Kim MS, et al. Feasibility of liquid biopsy using plasma and platelets for detection of anaplastic lymphoma kinase rearrangements in non-small cell lung cancer. J Cancer Res Clin Oncol, 2019, 145: 2071-2082. DOI: 10.1007/s00432-019-02944-w
|
| [114] |
Rachidi S, Metelli A, Riesenberg B, et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci Immunol, 2017, 2(11): eaai7911. DOI: 10.1126/sciimmunol.aai7911
|
| [115] |
Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer, 2017, 111: 176-181. DOI: 10.1016/j.lungcan.2017.07.024
|
| [116] |
Kim J, Yao F, Xiao Z, et al. MicroRNAs and metastasis: small RNAs play big roles. Cancer Metastasis Rev, 2018, 37: 5-15. DOI: 10.1007/s10555-017-9712-y
|
| [117] |
Solé C, Lawrie CH. MicroRNAs and metastasis. Cancers, 2019, 12(1): 96. DOI: 10.3390/cancers12010096
|
| [118] |
Han C, Yu Z, Duan Z, et al. Role of microRNA-1 in human cancer and its therapeutic potentials. BioMed Res Int, 2014, 2014.
|
| [119] |
Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med, 2009, 361(15): 1437-1447. DOI: 10.1056/NEJMoa0901282
|
| [120] |
Wang H, Peng R, Wang J, et al. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenetics, 2018, 10(1): 1-10. DOI: 10.1186/s13148-017-0434-3
|
| [121] |
Hu J, Cai G, Xu Y, et al. The Plasma microRNA miR-1914* and-1915 Suppresses Chemoresistant in Colorectal Cancer Patients by Down-regulating NFIX. Curr Mol Med, 2016, 16(1): 70-82. DOI: 10.2174/1566524016666151222144656
|
| [122] |
Zhou M, Liu Z, Zhao Y, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem, 2010, 285(28): 21496-21507. DOI: 10.1074/jbc.M109.083337
|
| [123] |
Giovannetti E, Funel N, Peters GJ, et al. MicroRNA-21 in Pancreatic Cancer: Correlation with Clinical Outcome and Pharmacologic Aspects Underlying Its Role in the Modulation of Gemcitabine ActivitymiR-21 and Gemcitabine Resistance in Pancreatic Cancer. Cancer Res, 2010, 70(11): 4528-4538. DOI: 10.1158/0008-5472.CAN-09-4467
|
| [124] |
Yuwen DL, Sheng BB, Liu J, et al. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur Rev Med Pharmacol Sci, 2017, 21(11): 2650-2658.
|
| [125] |
Mosakhani N, Sarhadi VK, Borze I, et al. MicroRNA profiling differentiates colorectal cancer according to KRAS status. Genes, Chromosomes Cancer, 2012, 51(1): 1-9. DOI: 10.1002/gcc.20925
|
| [126] |
Kato S, Schwaederlé MC, Fanta PT, et al. Genomic assessment of blood-derived circulating tumor DNA in patients with colorectal cancers: correlation with tissue sequencing, therapeutic response, and survival. JCO Precis Oncol, 2019, 3: 1-16. DOI: 10.1200/PO.18.00158
|
| [127] |
Li RY, Liang ZY. Circulating tumor DNA in lung cancer: real-time monitoring of disease evolution and treatment response. Chin Med J, 2020, 133(20): 2476-2485. DOI: 10.1097/CM9.0000000000001097
|
| [128] |
Zhong S, Golpon H, Zardo P, et al. miRNAs in lung cancer. A systematic review identifies predictive and prognostic miRNA candidates for precision medicine in lung cancer. Transl Res, 2021, 230: 164-196. DOI: 10.1016/j.trsl.2020.11.012
|
| [129] |
Kabzinski J, Maczynska M, Majsterek I. MicroRNA as a novel biomarker in the diagnosis of head and neck cancer. Biomolecules, 2021, 11(6): 844. DOI: 10.3390/biom11060844
|
| [130] |
Hoey C, Liu SK. Circulating blood miRNAs for prostate cancer risk stratification: miRroring the underlying tumor biology with liquid biopsies. Res Rep Urol, 2019: 29-42.
|
| [131] |
Yang C, Chen F, Wang S, et al. Circulating tumor cells in gastrointestinal cancers: current status and future perspectives. Front Oncol, 2019, 9: 1427. DOI: 10.3389/fonc.2019.01427
|
| [132] |
Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget, 2015, 6(13): 10697. DOI: 10.18632/oncotarget.4037
|
| [133] |
Tucci M, Mannavola F, Passarelli A, et al. Exosomes in melanoma: a role in tumor progression, metastasis and impaired immune system activity. Oncotarget, 2018, 9(29): 20826. DOI: 10.18632/oncotarget.24846
|
| [134] |
Xie J, Wei J, Lv L, et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal, 2020, 18(1): 1-13. DOI: 10.1186/s12964-019-0473-9
|
| [135] |
Qu Z, Wu J, Wu J, et al. Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget, 2017, 8(46): 80666. DOI: 10.18632/oncotarget.20881
|
| [136] |
Andreu Z, Oshiro RO, Redruello A, et al. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur J Pharm Sci, 2017, 98: 70-79. DOI: 10.1016/j.ejps.2016.10.008
|
| [137] |
Petrella G, Ciufolini G, Vago R, et al. Urinary metabolic markers of bladder cancer: A reflection of the tumor or the response of the body? Metabolites, 2021, 11(11): 756. DOI: 10.3390/metabo11110756
|
| [138] |
Sato T, Kawasaki Y, Maekawa M, et al. Accurate quantification of urinary metabolites for predictive models manifest clinicopathology of renal cell carcinoma. Cancer Sci, 2020, 111(7): 2570-2578. DOI: 10.1111/cas.14440
|
| [139] |
Birkenkamp-Demtröder K, Nordentoft I, Christensen E, et al. Genomic alterations in liquid biopsies from patients with bladder cancer. Eur Urol, 2016, 70(1): 75-82. DOI: 10.1016/j.eururo.2016.01.007
|
| [140] |
Asante DB, Calapre L, Ziman M, et al. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: ready for prime time? Cancer Lett, 2020, 468: 59-71. DOI: 10.1016/j.canlet.2019.10.014
|
| [141] |
Nijakowski K, Gruszczyński D, Kopała D, et al. Salivary metabolomics for oral squamous cell carcinoma diagnosis: a systematic review. Metabolites, 2022, 12(4): 294. DOI: 10.3390/metabo12040294
|
| [142] |
Takamori S, Ishikawa S, Suzuki J, et al. Differential diagnosis of lung cancer and benign lung lesion using salivary metabolites: A preliminary study. Thorac Cancer, 2022, 13(3): 460-465. DOI: 10.1111/1759-7714.14282
|
| [143] |
Li F, Yoshizawa JM, Kim KM, et al. Discovery and validation of salivary extracellular RNA biomarkers for noninvasive detection of gastric cancer. Clin Chem, 2018, 64(10): 1513-1521. DOI: 10.1373/clinchem.2018.290569
|
| [144] |
Lee YC, Kim SI, Kwon H, et al. Circulating tumor DNA in the saliva of patients with head and neck cancer: A pilot report. Oral Dis, 2021, 27(6): 1421-1425. DOI: 10.1111/odi.13683
|
| [145] |
Lee SH, Kim EY, Kim T, et al. Compared to plasma, bronchial washing fluid shows higher diagnostic yields for detecting EGFR-TKI sensitizing mutations by ddPCR in lung cancer. Resp Res, 2020, 21: 1-8. DOI: 10.1186/s12931-019-1261-1
|
| [146] |
Callejon-Leblic B, Garcia-Barrera T, Gravalos-Guzman J, et al. Metabolic profiling of potential lung cancer biomarkers using bronchoalveolar lavage fluid and the integrated direct infusion/gas chromatography mass spectrometry platform. J Proteomics, 2016, 145: 197-206. DOI: 10.1016/j.jprot.2016.05.030
|
| [147] |
Zhong CH, Tong D, Zhou ZQ, et al. Performance evaluation of detecting circulating tumor cells and tumor cells in bronchoalveolar lavage fluid in diagnosis of peripheral lung cancer. J Thorac Dis, 2018, 10(Suppl 7): S830. DOI: 10.21037/jtd.2017.12.125
|
| [148] |
Kim IA, Hur JY, Kim HJ, et al. Extracellular Vesicle-Based Bronchoalveolar Lavage Fluid Liquid Biopsy for EGFR Mutation Testing in Advanced Non-Squamous NSCLC. Cancers, 2022, 14(11): 2744. DOI: 10.3390/cancers14112744
|
| [149] |
Ruan H, Zhou Y, Shen J, et al. Circulating tumor cell characterization of lung cancer brain metastases in the cerebrospinal fluid through single‐cell transcriptome analysis. Clin Transl Med, 2020, 10(8): e246. DOI: 10.1002/ctm2.246
|
| [150] |
Escudero L, Llort A, Arias A, et al. Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat Commun, 2020, 11(1): 5376. DOI: 10.1038/s41467-020-19175-0
|
| [151] |
Bevacqua E, Farshchi J, Niklison-Chirou MV, et al. Role of MicroRNAs in the development and progression of the four medulloblastoma subgroups. Cancers, 2021, 13(24): 6323. DOI: 10.3390/cancers13246323
|
| [152] |
Shalaby T, Grotzer MA. Tumor-associated CSF MicroRNAs for the prediction and evaluation of CNS malignancies. Int J Mol Sci, 2015, 16(12): 29103-29119. DOI: 10.3390/ijms161226150
|
| [153] |
Tong L, Ding N, Tong X, et al. Tumor-derived DNA from pleural effusion supernatant as a promising alternative to tumor tissue in genomic profiling of advanced lung cancer. Theranostics, 2019, 9(19): 5532. DOI: 10.7150/thno.34070
|
| [154] |
Pérez-Barrios C, Sánchez-Herrero E, Garcia-Simón N, et al. ctDNA from body fluids is an adequate source for EGFR biomarker testing in advanced lung adenocarcinoma. Clin Chem Lab Med, 2021, 59(7): 1221-1229. DOI: 10.1515/cclm-2020-1465
|
| [155] |
Hamilton G, Hochmair M, Rath B, et al. Small cell lung cancer: Circulating tumor cells of extended stage patients express a mesenchymal-epithelial transition phenotype. Cell Adhes Migr, 2016, 10(4): 360-367. DOI: 10.1080/19336918.2016.1155019
|
| [156] |
Beije N, Kraan J, den Bakker MA, et al. Improved diagnosis and prognostication of patients with pleural malignant mesothelioma using biomarkers in pleural effusions and peripheral blood samples–a short report. Cell Oncol, 2017, 40: 511-519. DOI: 10.1007/s13402-017-0327-7
|
| [157] |
Liao J, Liu RAN, Shi YJ, et al. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int J Oncol, 2016, 48(6): 2567-2579. DOI: 10.3892/ijo.2016.3453
|
| [158] |
Vaksman O, Tropé C, Davidson B, et al. Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis, 2014, 35(9): 2113-2120. DOI: 10.1093/carcin/bgu130
|
| No. | Liquid Biopsies | Analyte | Application | Analytical Techniques | Cancer Diagnosed | References |
| ctDNA | Treatment strategy and responsiveness, Stratification of stages Diagnosis, | Real-time allele-specific PCR, sanger sequencing, TEC seq, NGS, whole-genome sequencing (WGS), ddPCR, whole-exome sequencing (WES) | NSCLC, Colorectal cancer, Breast cancer, Lung cancer | [33–35, 126,127] | ||
| 1 | Blood | miRNA | Diagnosis, prognosis, risk stratification | Microarray, qRT-PCR, ddPCR, NGS | Breast cancer, lung cancer, HNSCC, Prostate cancer | [36,128–130] |
| CTCs | Detection, treatment effectiveness, tumor relapse, drug resistance | Isolation-by-Size-of epithelial-Tumor (ISET) Methodology, Flow cytometry, CellSearch®, AdnaTest®, fluorescence in-situ hybridization (FISH) | Medulloblastoma, Gastrointestinal Cancers, Breast cancer, Colorectal cancer, Lung cancer, Prostate cancer, skin cancer | [37,131,132] | ||
| Exosomes | Diagnosis, tumor diagnosis, stratification of stages, | Proteomics, Immunoblotting, ELISA, qRT-PCR | Melanoma, Lung cancer, hepatocellular carcinoma | [133–135] | ||
| Urinary EVs | Diagnosis, disease progression | ddPCR, microarray, qRT-PCR, western blotting | Prostate cancer, bladder cancer | [38,136] | ||
| 2 | Urine | Metabolites | Diagnosis | Ultra-High Performance Liquid Chromatography (UHPLC) Quadrupole Time of Flight Mass Spectrometry (TOF-MS), LCMS, LCMS/MS | Endometrial cancer, HNSCC, renal cell carcinoma | [39,137,138] |
| ctDNA | Diagnosis, prognosis | ddPCR, WES, WGS, multiplex RTPCR | Bladder cancer, ovarian cancer | [139,140] | ||
| Metabolites | Diagnosis | NMR, MS, GC, HPLC, Capillary electrophoresis time-of-flight mass spectrometry | Oral squamous cell carcinoma (OSCC), Lung cancer | [141,142] | ||
| 3 | Saliva | miRNA | Diagnosis | Reverse transcriptase real-time- PCR (RT-qPCR) | Nasopharyngeal carcinoma, Gastric cancer | [45,143] |
| ctDNA | Diagnosis | NGS, WES, multiplex PCR, | HNSCC | [144] | ||
| DNA | Diagnosis | Sanger sequencing, qPCR, ddPCR | Lung cancer, NSCLC | [47,145] | ||
| 4 | BAL | Metabolites | Diagnosis | label-free mass spectrometry, direct infusion high-resolution mass spectrometry (DI-ESI-QTOF-MS), (GC-MS) | Lung cancer | [48,146] |
| CTCs | Diagnosis | FISH | Lung cancer | [147] | ||
| EVs | Diagnosis | qPCR | NSCLC | [148] | ||
| CTCs | Diagnosis, metastasis | CellSearch® system, Single-cell RNA sequencing | Leptomeningeal metastases (LM), lung cancer | [52,149] | ||
| 5 | CSF | ctDNA | Diagnosis, survival rate, characterization, monitoring | NGS, ddPCR, WES | Glioblastoma, medulloblastoma | [54,150] |
| miRNA | Disease progression and stratification, diagnosis | NGS qRT-PCR, microarrays | Medulloblastoma, Primary central nervous system lymphoma (PCNSL), Glioblastoma | [151,152] | ||
| ctDNA | Diagnosis | NGS, qRT-PCR, qPCR, | Lung cancer, lung adenocarcinoma | [153,154] | ||
| 6 | Pleural fluid | CTCs | Diagnosis, prognosis, mesenchymal-epithelial transition | Western blot arrays, cell search | Small cell lung cancer (SCLC), malignant mesothelioma | [155,156] |
| EVs | Diagnosis, prognosis | MicroRNA microarray, qRT-PCR | Esophageal cancer, ovarian cancer | [157,158] | ||
| The blood, saliva, urine, CSF, BAL, and pleural fluid are routinely used as a liquid biopsy and ctDNA, exosomes, CTCs, tumor-educated platelets (TEPs), and miRNA are used as targeted biomarkers. | ||||||